Automated sample preparation for vitamin D
Introduction
High throughput clinical laboratories require a reliable automation platform for vitamin D analysis that is compliant with IVD regulations, enables a full audit trail and is easy to use. Chromsystems and Hamilton have developed MassSTAR – a solution that meets all these requirements.
Vitamin D (cholecalciferol) deficiency leads to reduced calcium levels and impaired bone mineralisation. Associated symptoms are rickets in children and osteoporosis in adults. Patients with vitamin D deficiency show increased excretion of collagen crosslinks, indicating a process of bone resorption. Thus, vitamin D is a recognised biomarker for osteoporosis and osteopenia. Additionally, it plays a role in the immune response, cardiovascular diseases and certain cancers. Determining the vitamin D status is therefore important for several diagnostic purposes.
25-OH vitamin D3 is the primary metabolite in the human body and the direct precursor of the physiologically active form, 1,25-(OH)2 vitamin D3. The first-line treatment for vitamin D deficiency is supplementation with vitamin D3 or vitamin D2. Although less effective than D3, vitamin D2 is easier to synthesise and is widely used as a supplement outside the EU, particularly in the USA.
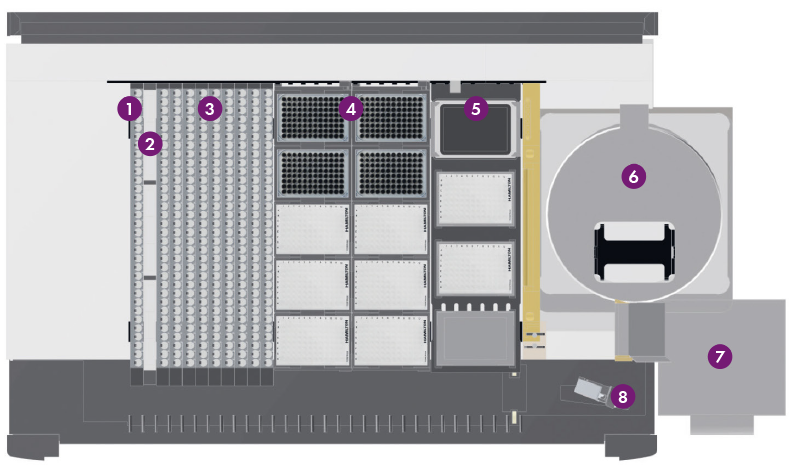

Figure 1: MassSTAR deck layout: 1 Carrier for calibrators and controls, 2 Carrier for reagents, 3 Carriers for sample tubes, 4 Carrier for tips and 96 well plates, 5 Carrier for (heater-) shaker and counterweight plates, 6 Centrifuge, 7 Waste, 8 Barcode reader
System description
The MassSTAR (Figure 1) is based on a Hamilton Microlab® STARlet with four channels, a CO-RE® Gripper, a barcode reader and an integrated centrifuge (Figure 2). The system deck consists of tube carriers for patient samples, calibrators, controls and reagents, as well as two carriers for 96 well plates and pipetting tips. Additionally, the deck includes a Hamilton Heater Shaker (HHS). Per run, up to 278 samples including calibrators and controls can be processed by the system.
The application is based on Hamilton’s STAR IVD Software. The method has been optimised to enable best performance. A user-friendly Graphical User Interface (GUI) makes the system easy to use and generates output files ready to use for all common LC-MS/MS systems.
Kit description
The Chromsystems reagent kit „MassChrom® 25-OH-Vitamin D3/D2 in serum/plasma” for automated sample preparation on MassSTAR (Chromsystems order no. 62000/1000/F) provides robust, precise and reproducible results.
The method is completely validated for the majority of tandem mass spectrometers on the market. Sample preparation is reduced to a simple and effective protein precipitation combined with an online purification step (trap column). Additionally, the isotopically labelled internal standards compensate all residual matrix effects.


Chromsystems MassChrom® 25-OH-Vitamin D3/D2 in Serum/Plasma Reagent Kit
Workflow
First, all resources are loaded and the barcodes of samples and plates are traced. In the default configuration, secondary sample tubes with dimensions of 13 x 75 mm or 17 x 100 mm are considered. A wide variety of other tube types can easily be added subsequently to accommodate the sample tubes used in the laboratory. After the transfer of 200 µL Internal Standard and 25 µL Precipitation Reagent, 100 µL of the premixed samples are added to the dedicated 96 well filter plate. Total Aspiration and Dispense Monitoring (TADM™) ensures proper sample and reagent pipetting. Erroneous transfers such as coagulated or foamy samples or samples with insufficient volumes are detected and flagged. If the error cannot be corrected by the autonomous error handling, they are excluded from further processing.
After sample transfer, the plate is agitated at 600 rpm for 10 min on the HHS and afterwards centrifuged for 5 min at 2000 g into a 96 well collection plate. The samples are then ready to be analysed by LC-MS/MS. For 96 samples, the entire sample preparation takes approximately 100 min.
Technology
One of the key safety features is the Total Aspiration and Dispense Monitoring TADM™, a pressure based technology by Hamilton. Each aspiration and dispensation step in each pipetting channel is monitored in real time. TADM™ verifies the sample transfer with a traceable digital audit trail and identifies errors such as foaming samples or incorrect volumes that have been transferred. A Graphical User Interface (GUI) guides the user through the loading process along with an inventory check (e.g. barcode matching, checking the correct positioning of the plates, verifying appropriate liquid levels for the reagents). This means that all reagents and consumables are available in sufficient quantities before the run is started, ensuring that no further user intervention is required, thereby providing additional walkaway time. Descriptive screens monitor the current state of the sample preparation process and show the steps the system will perform next. The GUI also prevents invalid user inputs and protects method files against unauthorised manipulation.
Results
The recovery in serum and plasma reached values between 106 and 111% for both analytes. The method‘s precision has been evaluated as inter- and intra-assay precision. Samples of two different concentration levels were prepared ten times and measured in a single sequence to determine intra-assay precision. Inter-assay precision was evaluated by preparing 100 samples of two different concentration levels on 10 different days. The coefficients of variation ranged between 2.4 and 4.9% (intra-assay) or between 4.3 and 8.2% (inter-assay), depending on respective analytes and concentration levels. Comprehensive data are available in the instruction manual.
Linearity was determined by spiking serum and plasma samples with defined amounts of authentic substances. The Lower Limit of Quantification (LLOQ) was evaluated by diluting a serum/plasma sample with analyte free matrix. Determinations with SCIEX Triple QuadTM 4500 mass spectrometer revealed that the method is linear from the LLOQ (1.0 µg/L) to the stated Upper Limit of Quantitation (250 µg/L).
Method comparison
59 real patient samples per analyte were collected and analysed using the Chromsystems reagent kit „MassChrom® 25-OH-Vitamin D3/D2 in serum/plasma” (Figure 2). For 25-OH-Vitamin D3, it was sufficient to spike half of the samples in order to cover the linear range between the LLOQ and ULOQ. To cover the entire range, all 25-OH vitamin D2 samples were spiked, as an analyte value around the LLOQ was expected in the majority of the samples. All samples were processed manually as well as by the MassSTAR and compared.
The results of the Passing-Bablok analysis demonstrate equivalence of sample preparation by the MassSTAR compared to the manual sample preparation. Over the entire concentration range, the slope was between 0.95 to 1.02 for both analytes.
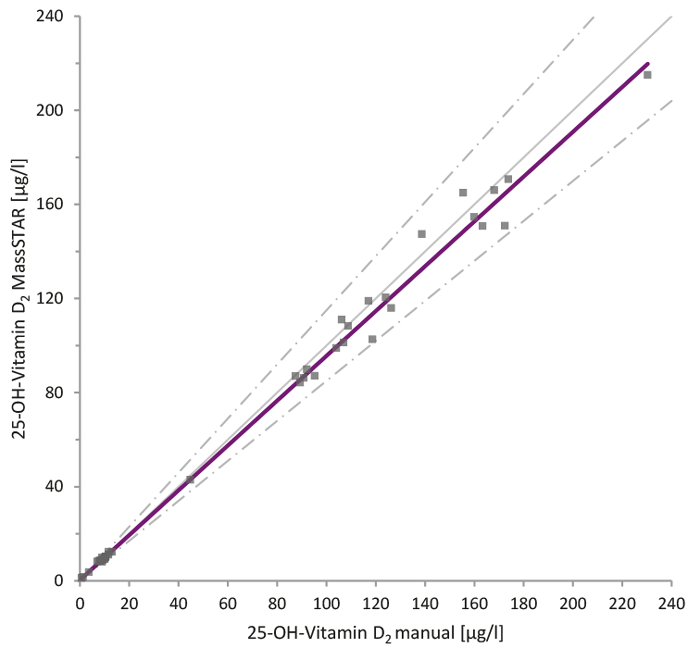

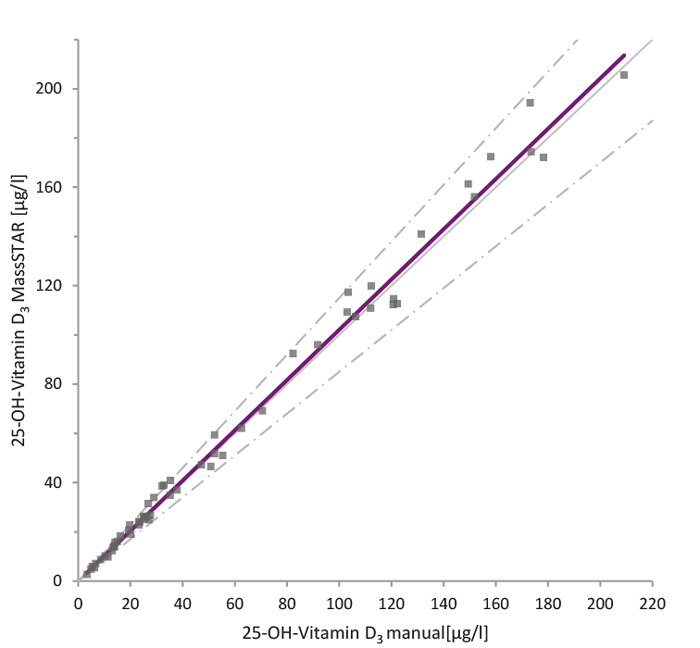

Figure 2: Comparison of manual and automated sample preparation for 25-OH-Vitamin D3/D2 analysis. Passing-Bablok analysis between manual and automated (MassSTAR) sample preparation methods for the analysis of 25-OH-Vitamin D2 (A) and D3 (B) using the Chromsystems MassChrom® reagent kit. A total of 59 real patient samples per analyte were analysed, with selective spiking to cover the full linear range.
Last Update 11th of November 2024
These products are not available in all countries.

