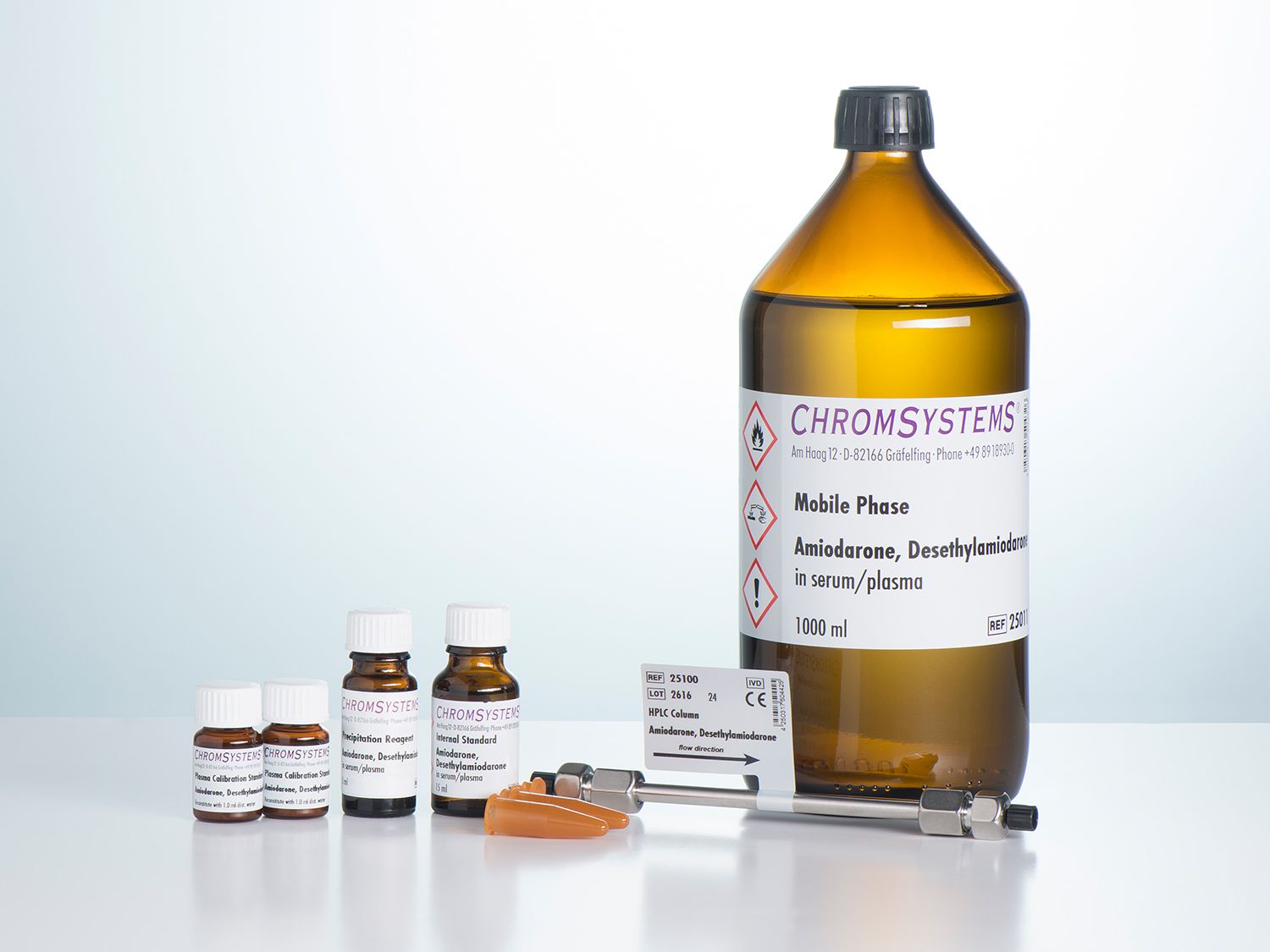
Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
Analysis of active metabolite included
Quick and easy sample preparation
Stable calibration standards and controls
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
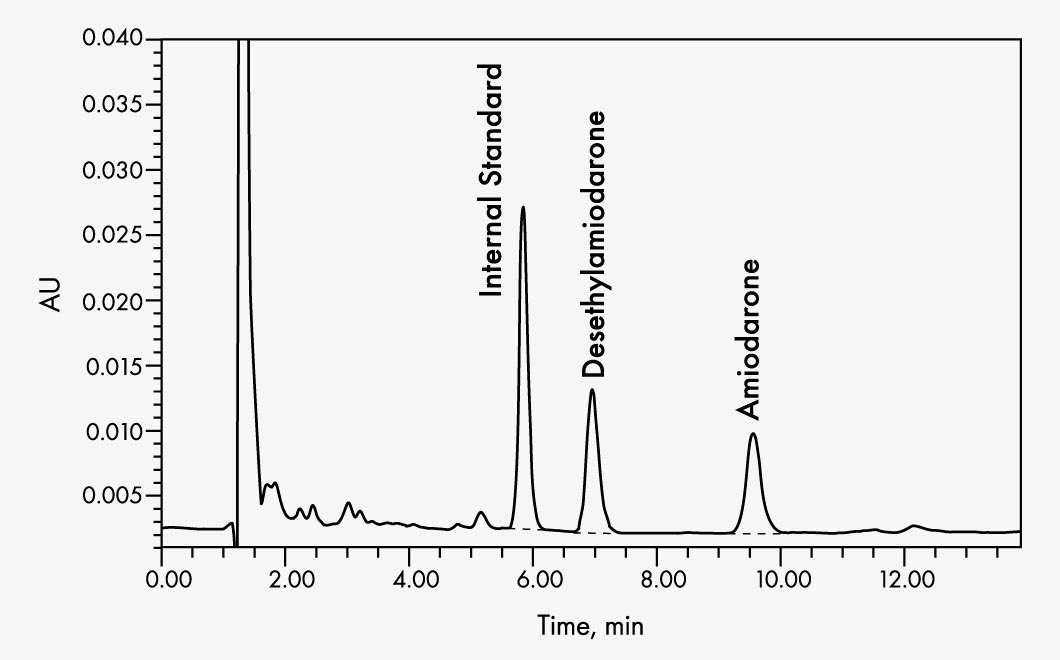

Amiodarone
Desethylamiodarone
Clinical relevance
Amiodarone is a typical representative of the class III antiarrhythmic agents that induce prolongation of the action potentials and refractory periods in the heart. It is approved in Germany for the treatment of various tachycardia arrhythmias, and is often prescribed in combination with other beta blockers. Additionally, amiodarone is often prescribed when other medications show no effect or stop working. Although amiodarone itself rarely causes cardiac arrhythmias, adverse effects may occur in other organs, e.g. reversible sensitivity to light in the eyes and the skin with risk of sunburn, increased liver-related values, as well as very rarely lung damages and hyperthyroidism/hypothyroidism. Regular monitoring of the amiodarone levels is therefore essential. Its bioactive metabolite desethylamiodarone can also be used for measurements.
Product advantages
- Metabolite analysis included
- Simple and fast sample preparation
- Stable internal standards and quality controls
This assay allows for the simple and reliable HPLC analysis of amiodarone and desethylamiodarone. Sample preparation is based on a simple and very efficient method of precipitation of all of the interfering components of the sample matrix. The separation is then performed on an isocratic HPLC system with UV detector.
The MassTox® TDM Series A Parameter Set for Antiarrhythmic Drugs is also available for using LC-MS/MS to analyse amiodarone and desethylamiodarone.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 0.15 mg/l (Amiodarone) |
| Upper Limit of Quantification | up to 20 mg/l |
| Intraassay | CV = 0.6–1.1 % |
| Interassay | CV = 3.4–5.5 % |
| Recovery | 99–106 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | approx. 11 min |
| Injection Volume | 25 µl |
| Flow Rate | 1 ml/min |
| Column Temperature | ambient (~ 25 °C) |
| Gradient | isocratic |
| Wavelengths | 242 nm |
| Additional Info | Any isocratic HPLC system with UV detector is suitable. |
| Parameters | Amiodarone, Desethylamiodarone |
-
 Amiodarone, Desethylamiodarone Plasma Calibration StandardOrder no.: 25005Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
Amiodarone, Desethylamiodarone Plasma Calibration StandardOrder no.: 25005Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
-
 HPLC Column Amiodarone and Desethylamiodarone in Serum/PlasmaOrder no.: 25100Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
HPLC Column Amiodarone and Desethylamiodarone in Serum/PlasmaOrder no.: 25100Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
-
 Amiodarone, Desethylamiodarone Plasma Calibration StandardOrder no.: 25005Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
Amiodarone, Desethylamiodarone Plasma Calibration StandardOrder no.: 25005Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
-
 HPLC Column Amiodarone and Desethylamiodarone in Serum/PlasmaOrder no.: 25100Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
HPLC Column Amiodarone and Desethylamiodarone in Serum/PlasmaOrder no.: 25100Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC


Amiodarone
Desethylamiodarone
Clinical relevance
Amiodarone is a typical representative of the class III antiarrhythmic agents that induce prolongation of the action potentials and refractory periods in the heart. It is approved in Germany for the treatment of various tachycardia arrhythmias, and is often prescribed in combination with other beta blockers. Additionally, amiodarone is often prescribed when other medications show no effect or stop working. Although amiodarone itself rarely causes cardiac arrhythmias, adverse effects may occur in other organs, e.g. reversible sensitivity to light in the eyes and the skin with risk of sunburn, increased liver-related values, as well as very rarely lung damages and hyperthyroidism/hypothyroidism. Regular monitoring of the amiodarone levels is therefore essential. Its bioactive metabolite desethylamiodarone can also be used for measurements.
Product advantages
- Metabolite analysis included
- Simple and fast sample preparation
- Stable internal standards and quality controls
This assay allows for the simple and reliable HPLC analysis of amiodarone and desethylamiodarone. Sample preparation is based on a simple and very efficient method of precipitation of all of the interfering components of the sample matrix. The separation is then performed on an isocratic HPLC system with UV detector.
The MassTox® TDM Series A Parameter Set for Antiarrhythmic Drugs is also available for using LC-MS/MS to analyse amiodarone and desethylamiodarone.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 0.15 mg/l (Amiodarone) |
| Upper Limit of Quantification | up to 20 mg/l |
| Intraassay | CV = 0.6–1.1 % |
| Interassay | CV = 3.4–5.5 % |
| Recovery | 99–106 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | approx. 11 min |
| Injection Volume | 25 µl |
| Flow Rate | 1 ml/min |
| Column Temperature | ambient (~ 25 °C) |
| Gradient | isocratic |
| Wavelengths | 242 nm |
| Additional Info | Any isocratic HPLC system with UV detector is suitable. |
| Parameters | Amiodarone, Desethylamiodarone |
-
 Amiodarone, Desethylamiodarone Plasma Calibration StandardOrder no.: 25005Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
Amiodarone, Desethylamiodarone Plasma Calibration StandardOrder no.: 25005Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
-
 HPLC Column Amiodarone and Desethylamiodarone in Serum/PlasmaOrder no.: 25100Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
HPLC Column Amiodarone and Desethylamiodarone in Serum/PlasmaOrder no.: 25100Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
-
 Amiodarone, Desethylamiodarone Plasma Calibration StandardOrder no.: 25005Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
Amiodarone, Desethylamiodarone Plasma Calibration StandardOrder no.: 25005Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
-
 HPLC Column Amiodarone and Desethylamiodarone in Serum/PlasmaOrder no.: 25100Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC
HPLC Column Amiodarone and Desethylamiodarone in Serum/PlasmaOrder no.: 25100Amiodarone and Desethylamiodarone in Serum/Plasma - HPLC