o-Cresol, p-Cresol and Phenol in Urine - HPLC
Easy sample preparation
Efficient acid hydrolysis
With custom-made internal standard
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
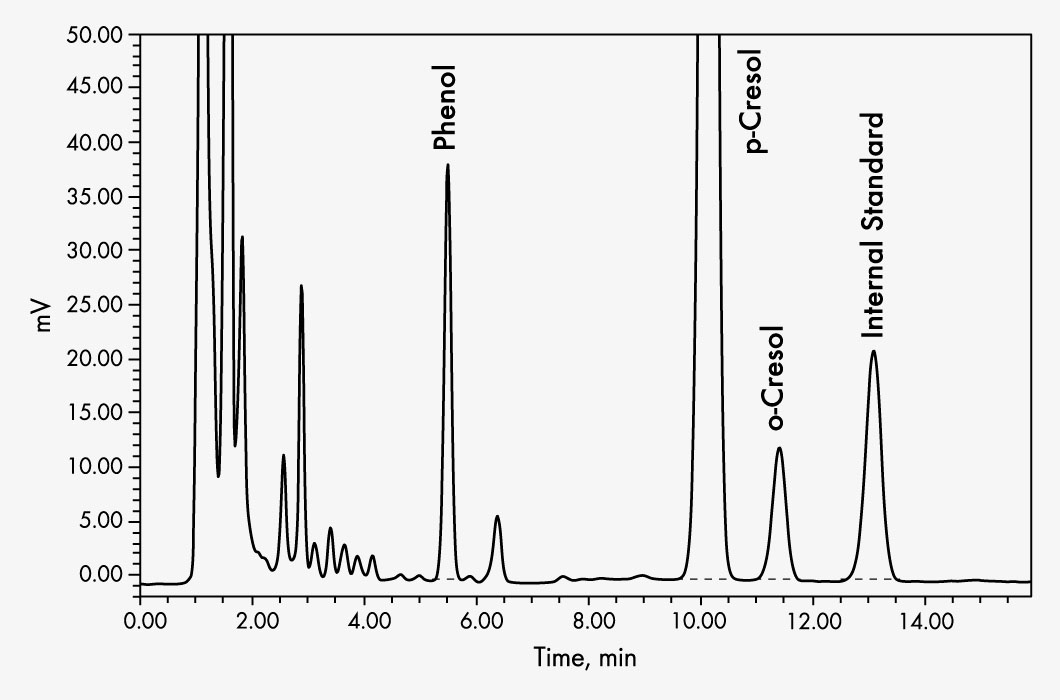

o-Cresol
p-Cresol
Phenol
Clinical relevance
Cresol and phenol are formed as metabolites in the body after exposure to toluene and benzene respectively. Toluene and benzene are toxic substances which are used on an industrial scale e.g. as solvents and fuel additives. Determination of the metabolite concentration in people exposed at work is performed as a measure for the occupational health monitoring of toluene and benzene exposure at work.
For any hazardous substance or its metabolite, a BTV (Biological Tolerance Value) is defined as a threshold value for maximum exposure, which assures safe working conditions without detriment to the health of the employee. If insufficient toxicological data is available to define a BTV, the so-called Biological Guideline Value (BGV) is suggested. With carcinogenic substances, generally no BTVs are defined, and the BGV should be used instead.
Product advantages
- Easy sample preparation
- Efficient acid hydrolysis
- With tailor-made internal standard
This Chromsystems assay allows for reliable and fast chromatographic determination of ortho- and para-cresol as well as phenol in an isocratic HPLC run. Sample preparation is fast and simple and requires only two steps: The first step is the acid hydrolysis, in which the phenols present in the form of sulphate and glucuronide conjugates are transformed efficiently into the free form.
The typical problems that occur in enzymatic cleavages, such as long reaction time or incomplete release, are avoided. In the second step, the samples are stabilised by adding a special buffer. The analytes are then measured using optimised chromatographic separation with highly sensitive fluorescence detection, so that potential interferences are mostly excluded. The inclusion of a tailor-made internal standard assures high precision and reliability in quantifying the analytes.
| Method of Analysis | HPLC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Tests | 100 | ||||||||||||
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. | ||||||||||||
| Lower Limit of Quantitation | 0.04 mg/l |
||||||||||||
| Upper Limit of Quantification | Phenol: at least to 50 mg/l |
||||||||||||
| Intraassay | CV ≤ 2.2 % |
||||||||||||
| Interassay | CV ≤ 3.8 % | ||||||||||||
| Recovery | 97–98 % | ||||||||||||
| Reference ranges | Occupational tolerance limits in urine
Reference range in urine (mg/g creatinine)
|
||||||||||||
| Specimen | Urine | ||||||||||||
| Sample Preparation |
*The hydrolysis vials are suitable as sample vials for a number of commonly used autosamplers. |
||||||||||||
| Run Time | 14 min | ||||||||||||
| Injection Volume | 10 µl | ||||||||||||
| Flow Rate | 1.3 ml/min | ||||||||||||
| Column Temperature | 30 °C | ||||||||||||
| Wavelengths | EX 270 nm, EM 300 nm | ||||||||||||
| Additional Info | Any isocratic HPLC system with fluorescence detector is suitable. | ||||||||||||
| Parameters | o-Cresol, p-Cresol, Phenol |
-
 o-Cresol, p-Cresol and Phenol Urine Calibration StandardOrder no.: 41003o-Cresol, p-Cresol and Phenol in Urine - HPLC
o-Cresol, p-Cresol and Phenol Urine Calibration StandardOrder no.: 41003o-Cresol, p-Cresol and Phenol in Urine - HPLC
-
 HPLC Column o-Cresol, p-Cresol and Phenol in UrineOrder no.: 41100o-Cresol, p-Cresol and Phenol in Urine - HPLC
HPLC Column o-Cresol, p-Cresol and Phenol in UrineOrder no.: 41100o-Cresol, p-Cresol and Phenol in Urine - HPLC -
 Hydrolysis Vials, with snap ring capsOrder no.: 41078Alternative for Hydrolysis Vials, with crimp caps (41077)
Hydrolysis Vials, with snap ring capsOrder no.: 41078Alternative for Hydrolysis Vials, with crimp caps (41077)
-
 o-Cresol, p-Cresol and Phenol Urine Calibration StandardOrder no.: 41003o-Cresol, p-Cresol and Phenol in Urine - HPLC
o-Cresol, p-Cresol and Phenol Urine Calibration StandardOrder no.: 41003o-Cresol, p-Cresol and Phenol in Urine - HPLC
-
 HPLC Column o-Cresol, p-Cresol and Phenol in UrineOrder no.: 41100o-Cresol, p-Cresol and Phenol in Urine - HPLC
HPLC Column o-Cresol, p-Cresol and Phenol in UrineOrder no.: 41100o-Cresol, p-Cresol and Phenol in Urine - HPLC


o-Cresol
p-Cresol
Phenol
Clinical relevance
Cresol and phenol are formed as metabolites in the body after exposure to toluene and benzene respectively. Toluene and benzene are toxic substances which are used on an industrial scale e.g. as solvents and fuel additives. Determination of the metabolite concentration in people exposed at work is performed as a measure for the occupational health monitoring of toluene and benzene exposure at work.
For any hazardous substance or its metabolite, a BTV (Biological Tolerance Value) is defined as a threshold value for maximum exposure, which assures safe working conditions without detriment to the health of the employee. If insufficient toxicological data is available to define a BTV, the so-called Biological Guideline Value (BGV) is suggested. With carcinogenic substances, generally no BTVs are defined, and the BGV should be used instead.
Product advantages
- Easy sample preparation
- Efficient acid hydrolysis
- With tailor-made internal standard
This Chromsystems assay allows for reliable and fast chromatographic determination of ortho- and para-cresol as well as phenol in an isocratic HPLC run. Sample preparation is fast and simple and requires only two steps: The first step is the acid hydrolysis, in which the phenols present in the form of sulphate and glucuronide conjugates are transformed efficiently into the free form.
The typical problems that occur in enzymatic cleavages, such as long reaction time or incomplete release, are avoided. In the second step, the samples are stabilised by adding a special buffer. The analytes are then measured using optimised chromatographic separation with highly sensitive fluorescence detection, so that potential interferences are mostly excluded. The inclusion of a tailor-made internal standard assures high precision and reliability in quantifying the analytes.
| Method of Analysis | HPLC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Tests | 100 | ||||||||||||
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. | ||||||||||||
| Lower Limit of Quantitation | 0.04 mg/l |
||||||||||||
| Upper Limit of Quantification | Phenol: at least to 50 mg/l |
||||||||||||
| Intraassay | CV ≤ 2.2 % |
||||||||||||
| Interassay | CV ≤ 3.8 % | ||||||||||||
| Recovery | 97–98 % | ||||||||||||
| Reference ranges | Occupational tolerance limits in urine
Reference range in urine (mg/g creatinine)
|
||||||||||||
| Specimen | Urine | ||||||||||||
| Sample Preparation |
*The hydrolysis vials are suitable as sample vials for a number of commonly used autosamplers. |
||||||||||||
| Run Time | 14 min | ||||||||||||
| Injection Volume | 10 µl | ||||||||||||
| Flow Rate | 1.3 ml/min | ||||||||||||
| Column Temperature | 30 °C | ||||||||||||
| Wavelengths | EX 270 nm, EM 300 nm | ||||||||||||
| Additional Info | Any isocratic HPLC system with fluorescence detector is suitable. | ||||||||||||
| Parameters | o-Cresol, p-Cresol, Phenol |
-
 o-Cresol, p-Cresol and Phenol Urine Calibration StandardOrder no.: 41003o-Cresol, p-Cresol and Phenol in Urine - HPLC
o-Cresol, p-Cresol and Phenol Urine Calibration StandardOrder no.: 41003o-Cresol, p-Cresol and Phenol in Urine - HPLC
-
 HPLC Column o-Cresol, p-Cresol and Phenol in UrineOrder no.: 41100o-Cresol, p-Cresol and Phenol in Urine - HPLC
HPLC Column o-Cresol, p-Cresol and Phenol in UrineOrder no.: 41100o-Cresol, p-Cresol and Phenol in Urine - HPLC -
 Hydrolysis Vials, with snap ring capsOrder no.: 41078Alternative for Hydrolysis Vials, with crimp caps (41077)
Hydrolysis Vials, with snap ring capsOrder no.: 41078Alternative for Hydrolysis Vials, with crimp caps (41077)
-
 o-Cresol, p-Cresol and Phenol Urine Calibration StandardOrder no.: 41003o-Cresol, p-Cresol and Phenol in Urine - HPLC
o-Cresol, p-Cresol and Phenol Urine Calibration StandardOrder no.: 41003o-Cresol, p-Cresol and Phenol in Urine - HPLC
-
 HPLC Column o-Cresol, p-Cresol and Phenol in UrineOrder no.: 41100o-Cresol, p-Cresol and Phenol in Urine - HPLC
HPLC Column o-Cresol, p-Cresol and Phenol in UrineOrder no.: 41100o-Cresol, p-Cresol and Phenol in Urine - HPLC