Parameter Set Mycophenolic Acid - LC-MS/MS
For the analysis of MPA and MPAG
3PLUS1® and 6PLUS1® Multilevel Calibrator Set and MassCheck® controls
Part of the continuously extended MassTox® TDM Series A
Assay validated according to IVDR (=> Declaration of Conformity)
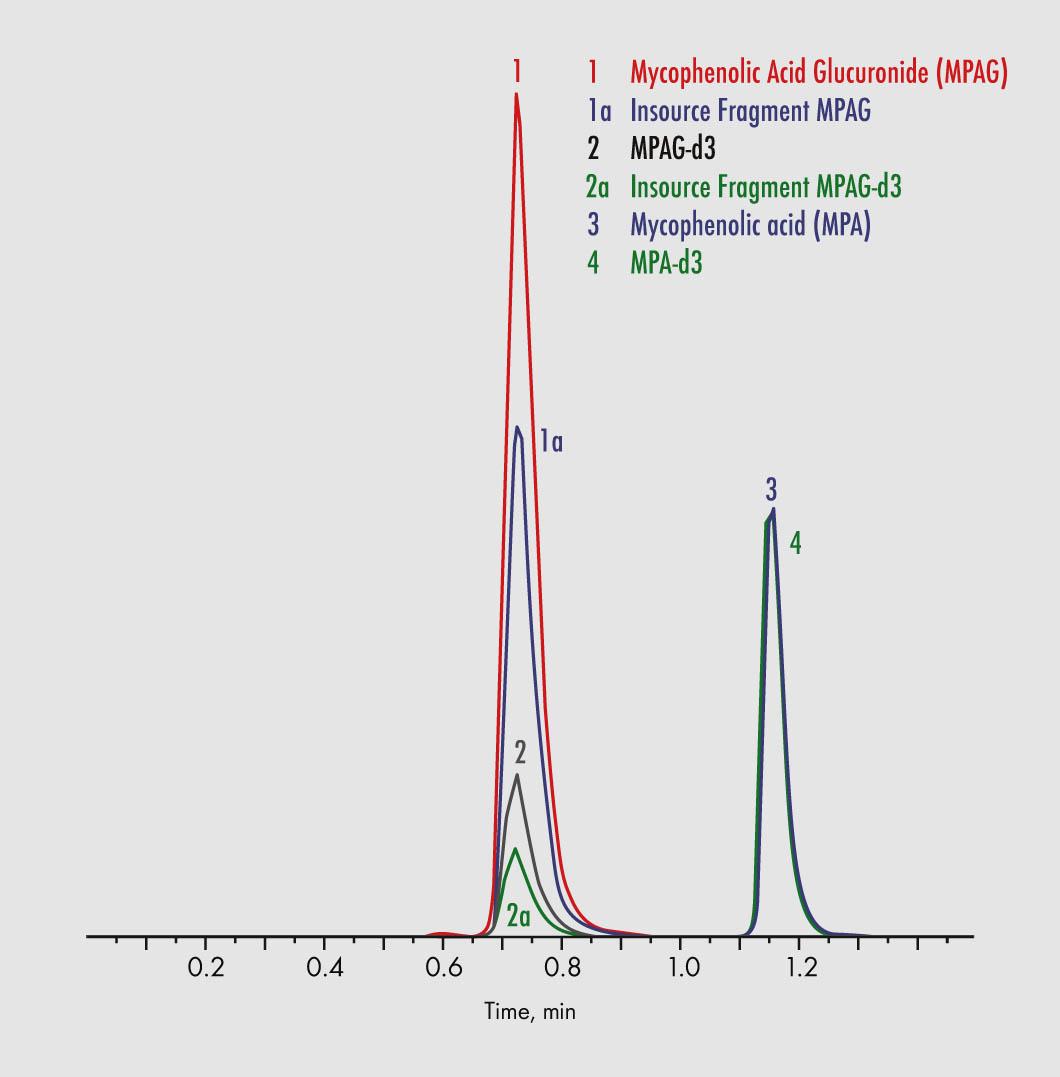

Mycophenolic Acid
Mycophenolic Acid Glucuronide
Clinical relevance
Mycophenolic acid (MPA) is used as an immunosuppressant agent to prevent organ transplant rejection. MPA selectively inhibits the synthesis of purines and specifically reduces the growth of B- and T-lymphocytes. Furthermore, MPA is also used to treat autoimmune diseases such as psoriasis, systemic lupus erythematosus or scleroderma.
Therapeutic monitoring of MPA in the blood is required to adjust individual concentrations within the therapeutic window and to attain a favourable ratio between therapeutic effects and side effects. Currently available drugs contain mycophenolate mofetil or mycophenolate sodium, which are both completely metabolised into the active metabolite MPA. For excretion, MPA is bound to glucuronic acid.
This parameter set allows the quantitative determination of mycophenolic acid and its metabolite mycophenolic acid glucuronide in human serum or plasma samples by LC-MS/MS.
It is intended as a therapeutic drug monitoring test, medically indicated for patients treated with mycophenolate mofetil or mycophenolate sodium.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
MassSTAR
There is also a CE-IVD compliant workflow method available using the MassSTAR to automate the sample preparation. => Parameter Set 92716
![]() More information about MassSTAR
More information about MassSTAR
Detailed performance evaluation data can be found in Appendices II and III of the instruction manual.
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data can be found in Appendices II and III of the instruction manual. |
| Lower and Upper Limit of Quantitation | MPA: 0.2 – 75 mg/l Different systems might show different performance data. |
| Specimen | Serum/Plasma |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual. Sample preparation with reaction vials: For the sample preparation with 96 well filter plates, please refer to the instruction manual. |
| Run Time | 1.8 min |
| Injection Volume | ≤ 25 µl (mass spectrometer dependent) |
| Flow Rate | 0.6 ml/min |
| Column Temperature | +20 to +25 °C |
| Gradient | binary |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Parameters | Mycophenolic Acid, Mycophenolic Acid Glucuronide |
92916 MassTox® TDM Parameter Set Mycophenolic Acid
| Order No. | MassTox® TDM Basic Kit A |
| 92111/200 | Basic Kit A for 200 tests |
| 92111/1000 | Basic Kit A for 1000 tests |
| 92111/1000/F | Basic Kit A for 1000 tests, for sample preparation with 96 well filter plates |
| Order No. | MassTox® TDM Basic Kit A (Quantities in kit for 200 tests) |
| 92001 | Mobile Phase 1, 1000 ml (1x) |
| 92002 | Mobile Phase 2, 1000 ml (1x) |
| 92003 | Precipitation Reagent, 50 ml (1x) |
| 92005 | Extraction Buffer, 5 ml (1x) |
| 92007 | Dilution Buffer 1, 50 ml (1x) |
| 92008 | Dilution Buffer 2, 50 ml (1x) |
| 92009 | Rinsing Solution, 1000 ml (1x) |
| 33006 | Reaction Vials, transparent, 1.5 ml, 100 pcs. (2x) |
| 92916 | MassTox® TDM Parameter Set Mycophenolic Acid/Glucuronide components |
| 46029 | 3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/Glucuronide, 4 x 1 ml (lyoph.) |
| 0235 | MassCheck® Mycophenolic Acid/Glucuronide Plasma Control, Level I, 5 x 1 ml (lyoph.) |
| 0236 | MassCheck® Mycophenolic Acid/Glucuronide Plasma Control, Level II, 5 x 1 ml (lyoph.) |
| 92246 | Internal Standard Set Mycophenolic Acid/Glucuronide, constisting of: Internal Standard Mix, 4 x 1 ml (lyoph.) Reconstitution Buffer, 5 ml |
| Order No. | Required components (not included in the kit or the parameter set) |
| 92110 | MassTox® TDM MasterColumn® A, equilibrated, with test chromatogram, 1 pc. |
| 92019 | Tuning Mix Mycophenolic Acid/Glucuronide (Analytes and Internal Standards), 1 ml |
| Order No. | Lab equipment |
| 15010 | PEEK Prefilter Housing, 1 pc. |
| 15011 | PEEK-encased Prefilter, 2 µm, 5 pcs. |
| 15070 | Stainless Steel Prefilter Housing, 1 pc. |
| 15071 | Stainless Steel Prefilter, 0.5 μm, 5 pcs. |
| 33006 | Reaction Vials, transparent, 1.5 ml, 100 pcs. |
-
 3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46029MassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46029MassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS -
 MassCheck® Mycophenolic Acid/Glucuronide Plasma ControlsOrder no.: 0234/0235/0236
MassCheck® Mycophenolic Acid/Glucuronide Plasma ControlsOrder no.: 0234/0235/0236MassTox® TDM Series A in Serum/Plasma – LC-MS/MS
-
 Internal Standard Set Mycophenolic Acid/GlucuronideOrder no.: 92246Component of the Parameter Set Mycophenolic Acid/Glucuronide, available separately
Internal Standard Set Mycophenolic Acid/GlucuronideOrder no.: 92246Component of the Parameter Set Mycophenolic Acid/Glucuronide, available separately
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Mycophenolic Acid/GlucuronideOrder no.: 92019Tuning Mix for the Parameter Set Mycophenolic Acid/Glucuronide - LC-MS/MS
Tuning Mix Mycophenolic Acid/GlucuronideOrder no.: 92019Tuning Mix for the Parameter Set Mycophenolic Acid/Glucuronide - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests with 96 Well Filter Plates - LC-MS/MSOrder no.: 92111/1000/F
Basic Kit A for 1000 Tests with 96 Well Filter Plates - LC-MS/MSOrder no.: 92111/1000/FPart of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phases
Sample preparation with 96 Well Filter PlatesValidated according to IVDR
-
 3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46029MassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46029MassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS -
 6PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46039/46039XLMassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46039/46039XLMassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS
-
 MassCheck® Mycophenolic Acid/Glucuronide Plasma ControlsOrder no.: 0234/0235/0236
MassCheck® Mycophenolic Acid/Glucuronide Plasma ControlsOrder no.: 0234/0235/0236MassTox® TDM Series A in Serum/Plasma – LC-MS/MS


Mycophenolic Acid
Mycophenolic Acid Glucuronide
Clinical relevance
Mycophenolic acid (MPA) is used as an immunosuppressant agent to prevent organ transplant rejection. MPA selectively inhibits the synthesis of purines and specifically reduces the growth of B- and T-lymphocytes. Furthermore, MPA is also used to treat autoimmune diseases such as psoriasis, systemic lupus erythematosus or scleroderma.
Therapeutic monitoring of MPA in the blood is required to adjust individual concentrations within the therapeutic window and to attain a favourable ratio between therapeutic effects and side effects. Currently available drugs contain mycophenolate mofetil or mycophenolate sodium, which are both completely metabolised into the active metabolite MPA. For excretion, MPA is bound to glucuronic acid.
This parameter set allows the quantitative determination of mycophenolic acid and its metabolite mycophenolic acid glucuronide in human serum or plasma samples by LC-MS/MS.
It is intended as a therapeutic drug monitoring test, medically indicated for patients treated with mycophenolate mofetil or mycophenolate sodium.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
MassSTAR
There is also a CE-IVD compliant workflow method available using the MassSTAR to automate the sample preparation. => Parameter Set 92716
![]() More information about MassSTAR
More information about MassSTAR
Detailed performance evaluation data can be found in Appendices II and III of the instruction manual.
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data can be found in Appendices II and III of the instruction manual. |
| Lower and Upper Limit of Quantitation | MPA: 0.2 – 75 mg/l Different systems might show different performance data. |
| Specimen | Serum/Plasma |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual. Sample preparation with reaction vials: For the sample preparation with 96 well filter plates, please refer to the instruction manual. |
| Run Time | 1.8 min |
| Injection Volume | ≤ 25 µl (mass spectrometer dependent) |
| Flow Rate | 0.6 ml/min |
| Column Temperature | +20 to +25 °C |
| Gradient | binary |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Parameters | Mycophenolic Acid, Mycophenolic Acid Glucuronide |
92916 MassTox® TDM Parameter Set Mycophenolic Acid
| Order No. | MassTox® TDM Basic Kit A |
| 92111/200 | Basic Kit A for 200 tests |
| 92111/1000 | Basic Kit A for 1000 tests |
| 92111/1000/F | Basic Kit A for 1000 tests, for sample preparation with 96 well filter plates |
| Order No. | MassTox® TDM Basic Kit A (Quantities in kit for 200 tests) |
| 92001 | Mobile Phase 1, 1000 ml (1x) |
| 92002 | Mobile Phase 2, 1000 ml (1x) |
| 92003 | Precipitation Reagent, 50 ml (1x) |
| 92005 | Extraction Buffer, 5 ml (1x) |
| 92007 | Dilution Buffer 1, 50 ml (1x) |
| 92008 | Dilution Buffer 2, 50 ml (1x) |
| 92009 | Rinsing Solution, 1000 ml (1x) |
| 33006 | Reaction Vials, transparent, 1.5 ml, 100 pcs. (2x) |
| 92916 | MassTox® TDM Parameter Set Mycophenolic Acid/Glucuronide components |
| 46029 | 3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/Glucuronide, 4 x 1 ml (lyoph.) |
| 0235 | MassCheck® Mycophenolic Acid/Glucuronide Plasma Control, Level I, 5 x 1 ml (lyoph.) |
| 0236 | MassCheck® Mycophenolic Acid/Glucuronide Plasma Control, Level II, 5 x 1 ml (lyoph.) |
| 92246 | Internal Standard Set Mycophenolic Acid/Glucuronide, constisting of: Internal Standard Mix, 4 x 1 ml (lyoph.) Reconstitution Buffer, 5 ml |
| Order No. | Required components (not included in the kit or the parameter set) |
| 92110 | MassTox® TDM MasterColumn® A, equilibrated, with test chromatogram, 1 pc. |
| 92019 | Tuning Mix Mycophenolic Acid/Glucuronide (Analytes and Internal Standards), 1 ml |
| Order No. | Lab equipment |
| 15010 | PEEK Prefilter Housing, 1 pc. |
| 15011 | PEEK-encased Prefilter, 2 µm, 5 pcs. |
| 15070 | Stainless Steel Prefilter Housing, 1 pc. |
| 15071 | Stainless Steel Prefilter, 0.5 μm, 5 pcs. |
| 33006 | Reaction Vials, transparent, 1.5 ml, 100 pcs. |
-
 3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46029MassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46029MassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS -
 MassCheck® Mycophenolic Acid/Glucuronide Plasma ControlsOrder no.: 0234/0235/0236
MassCheck® Mycophenolic Acid/Glucuronide Plasma ControlsOrder no.: 0234/0235/0236MassTox® TDM Series A in Serum/Plasma – LC-MS/MS
-
 Internal Standard Set Mycophenolic Acid/GlucuronideOrder no.: 92246Component of the Parameter Set Mycophenolic Acid/Glucuronide, available separately
Internal Standard Set Mycophenolic Acid/GlucuronideOrder no.: 92246Component of the Parameter Set Mycophenolic Acid/Glucuronide, available separately
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Mycophenolic Acid/GlucuronideOrder no.: 92019Tuning Mix for the Parameter Set Mycophenolic Acid/Glucuronide - LC-MS/MS
Tuning Mix Mycophenolic Acid/GlucuronideOrder no.: 92019Tuning Mix for the Parameter Set Mycophenolic Acid/Glucuronide - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests with 96 Well Filter Plates - LC-MS/MSOrder no.: 92111/1000/F
Basic Kit A for 1000 Tests with 96 Well Filter Plates - LC-MS/MSOrder no.: 92111/1000/FPart of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phases
Sample preparation with 96 Well Filter PlatesValidated according to IVDR
-
 3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46029MassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46029MassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS -
 6PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46039/46039XLMassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Mycophenolic Acid/GlucuronideOrder no.: 46039/46039XLMassTox® TDM Series A Mycophenolic Acid in Serum/Plasma – LC-MS/MS
-
 MassCheck® Mycophenolic Acid/Glucuronide Plasma ControlsOrder no.: 0234/0235/0236
MassCheck® Mycophenolic Acid/Glucuronide Plasma ControlsOrder no.: 0234/0235/0236MassTox® TDM Series A in Serum/Plasma – LC-MS/MS






