Levetiracetam (Keppra®) in Serum/Plasma - HPLC
Completes the analysis of antiepileptic drugs
High sample throughput
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
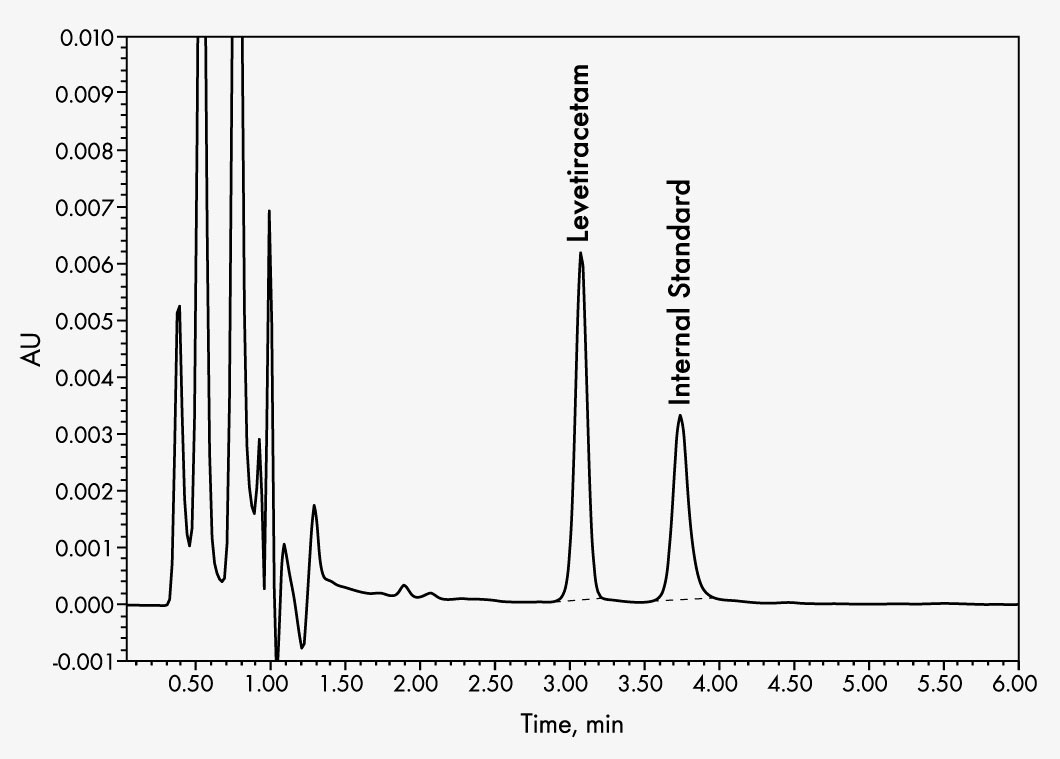

Levetiracetam (Keppra®)
Clinical relevance
Levetiracetam (Keppra®) is an antiepileptic that bears no structural similarity to other drugs. In the cells of the brain, Levetiracetam acts to provide presynaptic inhibition over multiple cascades of postsynaptic chloride ion influx.
It is approved for monotherapy of certain forms of epilepsy in patients of age 16 years and older. It may also be prescribed as add-on therapy for younger patients, e.g. for juvenile myoclonic epilepsy (also called Janz syndrome). The determination of the serum concentration of levetiracetam is important since it undergoes individual fluctuations which vary from patient to patient.
Product advantages
- Completion of the antiepileptic analysis
- Exact quantification with a tailored internal standard
This assay allows for the rapid and reliable determination of levetiracetam levels in serum or plasma. Sample preparation is based on an efficient and fast method of solid phase extraction (SPE). A tailored internal standard is used to achieve an exact quantification. This kit enables isocratic elution for chromatographic separation on an RP column with subsequent UV detection.
Another HPLC assay (order no. 22000) that can determine further antiepileptics in serum/plasma is also available. Additionally a TDM Parameter Set from the MassTox® TDM Series A is available that uses mass spectrometry for analysis and which can measure 26 antiepileptics.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 0.5 mg/l |
| Upper Limit of Quantification | up to 1000 mg/l |
| Intraassay | CV ≤ 1.3 % |
| Interassay | CV ≤ 3.7 % |
| Recovery | 90 % |
| Specimen | Serum/Plasma |
| Sample Preparation | Extraction:
Wash:
Elution:
|
| Run Time | 7 min |
| Injection Volume | 10 µl |
| Flow Rate | 1.2 ml/min |
| Column Temperature | max. + 25 °C |
| Gradient | isocratic |
| Wavelengths | 210 nm |
| Additional Info | Any isocratic HPLC system with UV detector is suitable. |
| Parameters | Levetiracetam |
-
 HPLC Column Levetiracetam (Keppra®) in Serum/PlasmaOrder no.: 24100
HPLC Column Levetiracetam (Keppra®) in Serum/PlasmaOrder no.: 24100Levetiracetam (Keppra®) in Serum/Plasma - HPLC
-
 HPLC Column Levetiracetam (Keppra®) in Serum/PlasmaOrder no.: 24100
HPLC Column Levetiracetam (Keppra®) in Serum/PlasmaOrder no.: 24100Levetiracetam (Keppra®) in Serum/Plasma - HPLC


Levetiracetam (Keppra®)
Clinical relevance
Levetiracetam (Keppra®) is an antiepileptic that bears no structural similarity to other drugs. In the cells of the brain, Levetiracetam acts to provide presynaptic inhibition over multiple cascades of postsynaptic chloride ion influx.
It is approved for monotherapy of certain forms of epilepsy in patients of age 16 years and older. It may also be prescribed as add-on therapy for younger patients, e.g. for juvenile myoclonic epilepsy (also called Janz syndrome). The determination of the serum concentration of levetiracetam is important since it undergoes individual fluctuations which vary from patient to patient.
Product advantages
- Completion of the antiepileptic analysis
- Exact quantification with a tailored internal standard
This assay allows for the rapid and reliable determination of levetiracetam levels in serum or plasma. Sample preparation is based on an efficient and fast method of solid phase extraction (SPE). A tailored internal standard is used to achieve an exact quantification. This kit enables isocratic elution for chromatographic separation on an RP column with subsequent UV detection.
Another HPLC assay (order no. 22000) that can determine further antiepileptics in serum/plasma is also available. Additionally a TDM Parameter Set from the MassTox® TDM Series A is available that uses mass spectrometry for analysis and which can measure 26 antiepileptics.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 0.5 mg/l |
| Upper Limit of Quantification | up to 1000 mg/l |
| Intraassay | CV ≤ 1.3 % |
| Interassay | CV ≤ 3.7 % |
| Recovery | 90 % |
| Specimen | Serum/Plasma |
| Sample Preparation | Extraction:
Wash:
Elution:
|
| Run Time | 7 min |
| Injection Volume | 10 µl |
| Flow Rate | 1.2 ml/min |
| Column Temperature | max. + 25 °C |
| Gradient | isocratic |
| Wavelengths | 210 nm |
| Additional Info | Any isocratic HPLC system with UV detector is suitable. |
| Parameters | Levetiracetam |
-
 HPLC Column Levetiracetam (Keppra®) in Serum/PlasmaOrder no.: 24100
HPLC Column Levetiracetam (Keppra®) in Serum/PlasmaOrder no.: 24100Levetiracetam (Keppra®) in Serum/Plasma - HPLC
-
 HPLC Column Levetiracetam (Keppra®) in Serum/PlasmaOrder no.: 24100
HPLC Column Levetiracetam (Keppra®) in Serum/PlasmaOrder no.: 24100Levetiracetam (Keppra®) in Serum/Plasma - HPLC