VMA, HVA, 5-HIAA in Urine - HPLC
No pH-adjustment necessary
Two internal standards available
Suitable for semi-automated sample preparation
Assay validated according to IVDR (=> Declaration of Conformity)
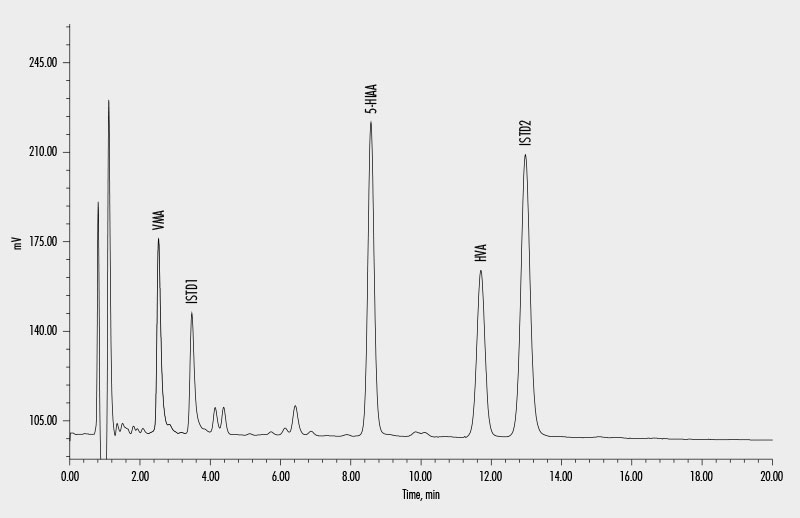

Vanillylmandelic Acid (VMA)
Homovanillic Acid (HVA)
5-Hydroxyindoleacetic Acid (5-HIAA)
Clinical relevance
This assay allows the simultaneous quantitative determination of vanillylmandelic acid (VMA), homovanillic acid (HVA), and 5-hydroxyindoleacetic acid (5-HIAA) in human urine samples by HPLC.
It is intended to be used for patients in whom the urinary levels of both vanillylmandelic acid (VMA) and homovanillic acid (HVA) are of clinical importance, primarily as an aid to diagnosis and monitoring of suspected neuroblastoma.
Furthermore, it is intended to be used for patients in whom the urinary level of 5-hydroxyindoleacetic acid (5-HIAA) is of clinical importance, primarily as an aid to diagnosis and monitoring of suspected carcinoids and serotonin-secreting neuroendocrine tumours.
Assay characteristics
Sample preparation consists of an efficient solid-phase extraction, which can be performed manually or semi-automated with an Gilson® ASPEC® liquid handler. The analytes and internal standards are then chromatographically separated in a single isocratic HPLC run and quantified using an electrochemical detector (ECD). The use of two internal standards ensures high precision and reliability in quantitation of the analytes.
Detailed performance evaluation data can be found in Appendices II and III of the instruction manual.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data can be found in Appendices II and III of the instruction manual. |
| Lower and Upper Limit of Quantitation | VMA: 0.32 mg/L – 80 mg/L The LLOQ depends on the conditions of the working electrode. |
| Specimen | 24-hour urine. Alternatively spot urine may also be used. In this case, results must be related to creatinine. |
| Sample Preparation | The information on the manual sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual. Buffering of the urine sample/calibrator/control Sample extraction with Sample Clean Up Columns Continued sample preparation prior to injection
For the semi-automated sample preparation with the Gilson® ASPEC®, please refer to the instruction manual. |
| Run Time | 20 min |
| Injection Volume | 10-20 µL |
| Flow Rate | 1 mL/min |
| Column Temperature | +20 to +25 °C |
| Potential | approx. +700 mV to +850 mV |
| Additional Info | For the HPLC analysis of VMA, HVA and 5-HIAA in urine any isocratic HPLC system with electrochemical detector is suitable. |
| Parameters | 5-Hydroxyindoleacetic Acid (5-HIAA), Homovanillic Acid (HVA), Vanillylmandelic Acid (VMA) |


Vanillylmandelic Acid (VMA)
Homovanillic Acid (HVA)
5-Hydroxyindoleacetic Acid (5-HIAA)
Clinical relevance
This assay allows the simultaneous quantitative determination of vanillylmandelic acid (VMA), homovanillic acid (HVA), and 5-hydroxyindoleacetic acid (5-HIAA) in human urine samples by HPLC.
It is intended to be used for patients in whom the urinary levels of both vanillylmandelic acid (VMA) and homovanillic acid (HVA) are of clinical importance, primarily as an aid to diagnosis and monitoring of suspected neuroblastoma.
Furthermore, it is intended to be used for patients in whom the urinary level of 5-hydroxyindoleacetic acid (5-HIAA) is of clinical importance, primarily as an aid to diagnosis and monitoring of suspected carcinoids and serotonin-secreting neuroendocrine tumours.
Assay characteristics
Sample preparation consists of an efficient solid-phase extraction, which can be performed manually or semi-automated with an Gilson® ASPEC® liquid handler. The analytes and internal standards are then chromatographically separated in a single isocratic HPLC run and quantified using an electrochemical detector (ECD). The use of two internal standards ensures high precision and reliability in quantitation of the analytes.
Detailed performance evaluation data can be found in Appendices II and III of the instruction manual.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data can be found in Appendices II and III of the instruction manual. |
| Lower and Upper Limit of Quantitation | VMA: 0.32 mg/L – 80 mg/L The LLOQ depends on the conditions of the working electrode. |
| Specimen | 24-hour urine. Alternatively spot urine may also be used. In this case, results must be related to creatinine. |
| Sample Preparation | The information on the manual sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual. Buffering of the urine sample/calibrator/control Sample extraction with Sample Clean Up Columns Continued sample preparation prior to injection
For the semi-automated sample preparation with the Gilson® ASPEC®, please refer to the instruction manual. |
| Run Time | 20 min |
| Injection Volume | 10-20 µL |
| Flow Rate | 1 mL/min |
| Column Temperature | +20 to +25 °C |
| Potential | approx. +700 mV to +850 mV |
| Additional Info | For the HPLC analysis of VMA, HVA and 5-HIAA in urine any isocratic HPLC system with electrochemical detector is suitable. |
| Parameters | 5-Hydroxyindoleacetic Acid (5-HIAA), Homovanillic Acid (HVA), Vanillylmandelic Acid (VMA) |