Crosslinks in Urine - HPLC
Hydrolysis resistant internal standard
Easy sample preparation
Accurate and reliable calibration
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746

Deoxypyridinoline (DPD)
Pyridinoline (PYD)
Clinical relevance
Pyridinoline (PYD) and deoxypyridinoline (DPD) are produced to provide structural stability in the form of crosslinks when collagen fibrils are created. In pathological bone degeneration, the concentrations of PYD and DPD increase in the urine. This is the case with osteoporosis or various bone cancers, for example. Thus PYD and DPD are direct biomarkers for diagnosing post-menopausal osteoporosis and monitoring therapy after intake of drugs. A significant drop in crosslinks in the urine can be observed after just a few months of successful treatment. Furthermore, crosslink concentrations in the urine are subject to ongoing monitoring in patients at greater risk of fracture, so that suitable measures can be taken right away if the levels are already high.
Product advantages
- Hydrolysis-resistant internal standard
- Easy sample preparation
- Accurate and reliable calibration
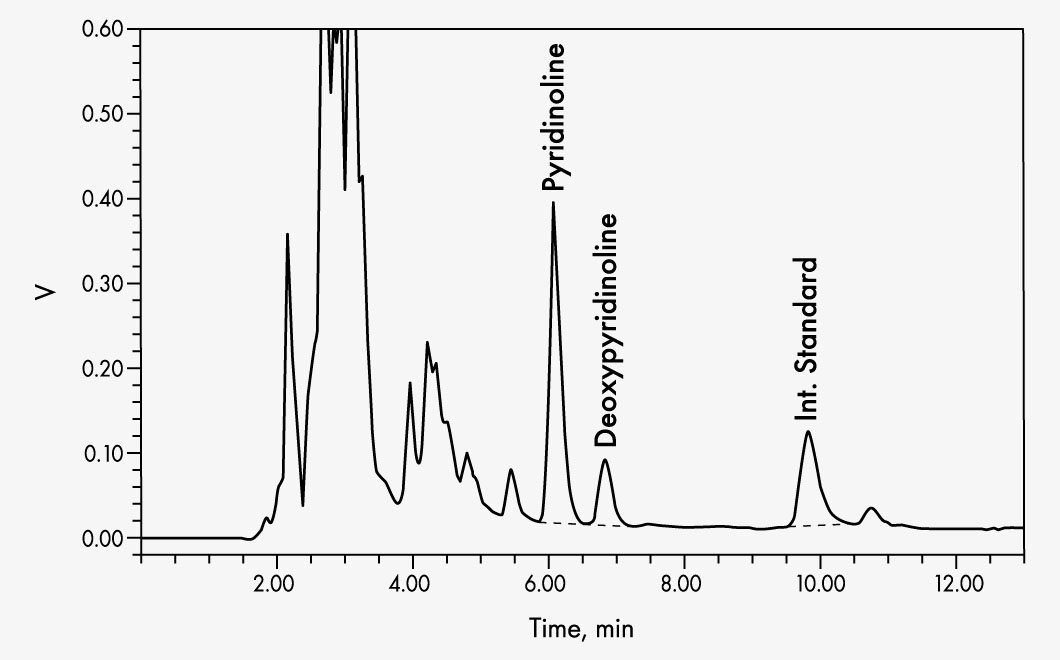
This kit allows simple and reliable simultaneous quantification of pyridinoline (PYD) and deoxypyridinoline (DPD) in urine using a simple isocratic HPLC system. Chromatographic separation is performed at an RP column with subsequent fluorescence detection. The use of an acidic hydrolysis-resistant internal standard maximises the precision and reliability of the determination. Interfering fluorophores are removed using solid phase extraction, and then PYD, DPD, and internal standard are eluted in one run.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | Pyridinoline: 15 pmol/ml |
| Upper Limit of Quantification | Pyridinoline: up to 3200 pmol/ml |
| Intraassay | Pyridinoline: CV = 3.8 % |
| Interassay | Pyridinoline: CV = 5.1 % |
| Recovery | Pyridinoline: 95.5 % (CV: 1.4 %); Deoxypyridinoline: 93.6 % (CV: 2.0 %) |
| Specimen | Spontaneous urine or collected for a period |
| Sample Preparation |
|
| Run Time | < 15 min |
| Injection Volume | 50 µl |
| Flow Rate | 1.2 ml/min |
| Column Temperature | ambient (~ 25 °C) |
| Gradient | isocratic |
| Wavelengths | EX 290 nm |
| Additional Info | Any isocratic HPLC system with fluorescence detector is suitable. |
| Parameters | Deoxypyridinoline (DPD), Pyridinoline (PYD) |


Deoxypyridinoline (DPD)
Pyridinoline (PYD)
Clinical relevance
Pyridinoline (PYD) and deoxypyridinoline (DPD) are produced to provide structural stability in the form of crosslinks when collagen fibrils are created. In pathological bone degeneration, the concentrations of PYD and DPD increase in the urine. This is the case with osteoporosis or various bone cancers, for example. Thus PYD and DPD are direct biomarkers for diagnosing post-menopausal osteoporosis and monitoring therapy after intake of drugs. A significant drop in crosslinks in the urine can be observed after just a few months of successful treatment. Furthermore, crosslink concentrations in the urine are subject to ongoing monitoring in patients at greater risk of fracture, so that suitable measures can be taken right away if the levels are already high.
Product advantages
- Hydrolysis-resistant internal standard
- Easy sample preparation
- Accurate and reliable calibration
This kit allows simple and reliable simultaneous quantification of pyridinoline (PYD) and deoxypyridinoline (DPD) in urine using a simple isocratic HPLC system. Chromatographic separation is performed at an RP column with subsequent fluorescence detection. The use of an acidic hydrolysis-resistant internal standard maximises the precision and reliability of the determination. Interfering fluorophores are removed using solid phase extraction, and then PYD, DPD, and internal standard are eluted in one run.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | Pyridinoline: 15 pmol/ml |
| Upper Limit of Quantification | Pyridinoline: up to 3200 pmol/ml |
| Intraassay | Pyridinoline: CV = 3.8 % |
| Interassay | Pyridinoline: CV = 5.1 % |
| Recovery | Pyridinoline: 95.5 % (CV: 1.4 %); Deoxypyridinoline: 93.6 % (CV: 2.0 %) |
| Specimen | Spontaneous urine or collected for a period |
| Sample Preparation |
|
| Run Time | < 15 min |
| Injection Volume | 50 µl |
| Flow Rate | 1.2 ml/min |
| Column Temperature | ambient (~ 25 °C) |
| Gradient | isocratic |
| Wavelengths | EX 290 nm |
| Additional Info | Any isocratic HPLC system with fluorescence detector is suitable. |
| Parameters | Deoxypyridinoline (DPD), Pyridinoline (PYD) |