Antibiotics in Serum/Plasma - HPLC
Simple to perform tests
One column and one sample prep for all parameters
Stable calibrators and controls available
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
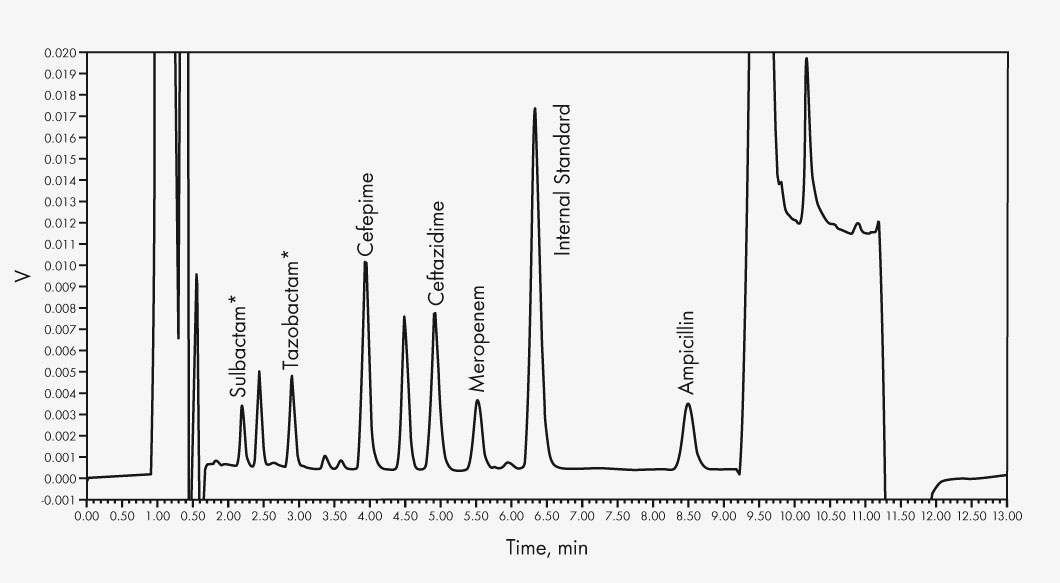

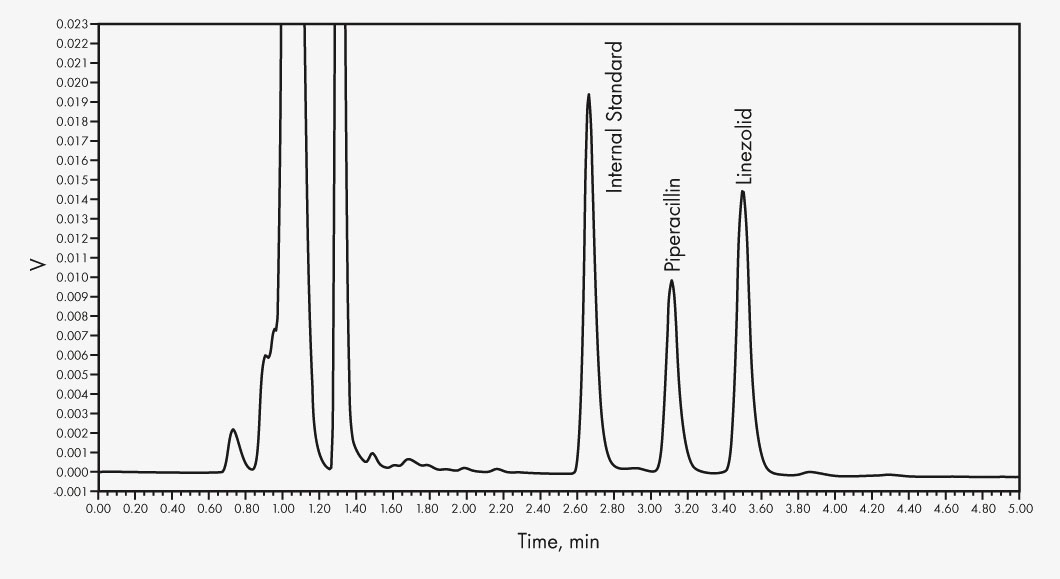

Ampicillin
Cefepime
Ceftazidime
Linezolid
Meropenem
Piperacillin
Sulbactam*
Tazobactam*
* For performance evaluation only
The assay Antibiotics in Serum/Plasma is an in vitro diagnostic product for the use in clinical laboratories. It is used for the quantitative determination of ampicillin, cefepime, ceftazidime, linezolid, meropenem and piperacillin in human serum and plasma samples for monitoring patients’ blood levels of these antibiotics. The calibrators and controls also include Sulbactam* and Tazobactam* for obtaining qualitative data (customer validation required).
The HPLC determination with subsequent UV detection is done in two groups, the first run via a mobile phase gradient, the second run isocratically. Two internal standards and stable matrix products ensure precise quantification. The additionally included Priming Solution increases sample stability notably.
* Qualitative information on Sulbactam and Tazobactam can be obtained, but data are not CE-IVD compliant.
Further Information: Therapeutic Drug Monitoring in Intensive Care
Read more about the importance of appropriate drug concentrations in antibiotic therapies to and help prevent over- and underdosing. This approach also reduces the risk of new multidrug resistant strains emerging and helps to provide the most effective drug dose to the patient.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 0.5–2.2 mg/l |
| Upper Limit of Quantification | up to 60–400 mg/l |
| Intraassay | CV = 0.5–1.6 % |
| Interassay | CV = 2.5–5.5 % |
| Recovery | 91–104 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 13 min (Group 1), 5 min (Group 2) |
| Injection Volume | 5 μl (Group 1), 10 μl (Group 2) |
| Flow Rate | 1 ml/min |
| Column Temperature | 22 °C |
| Gradient | binary (Group 1) |
| Wavelengths | 0–5.9 min 290 nm, then 210 nm (Group 1) |
| Additional Info | A binary HPLC gradient system with coolable injector, coolable column oven and UV-detector is required. |
| Information | Autosampler temperature: 10 °C |
| Parameters | Ampicillin, Cefepime, Ceftazidime, Linezolid, Meropenem, Piperacillin, Sulbactam, Tazobactam |
-
 3PLUS1® Multilevel Plasma Calibrator Set AntibioticsOrder no.: 61028Antibiotics in Serum/Plasma – HPLC
3PLUS1® Multilevel Plasma Calibrator Set AntibioticsOrder no.: 61028Antibiotics in Serum/Plasma – HPLC
-
 3PLUS1® Multilevel Plasma Calibrator Set AntibioticsOrder no.: 61028Antibiotics in Serum/Plasma – HPLC
3PLUS1® Multilevel Plasma Calibrator Set AntibioticsOrder no.: 61028Antibiotics in Serum/Plasma – HPLC




Ampicillin
Cefepime
Ceftazidime
Linezolid
Meropenem
Piperacillin
Sulbactam*
Tazobactam*
* For performance evaluation only
The assay Antibiotics in Serum/Plasma is an in vitro diagnostic product for the use in clinical laboratories. It is used for the quantitative determination of ampicillin, cefepime, ceftazidime, linezolid, meropenem and piperacillin in human serum and plasma samples for monitoring patients’ blood levels of these antibiotics. The calibrators and controls also include Sulbactam* and Tazobactam* for obtaining qualitative data (customer validation required).
The HPLC determination with subsequent UV detection is done in two groups, the first run via a mobile phase gradient, the second run isocratically. Two internal standards and stable matrix products ensure precise quantification. The additionally included Priming Solution increases sample stability notably.
* Qualitative information on Sulbactam and Tazobactam can be obtained, but data are not CE-IVD compliant.
Further Information: Therapeutic Drug Monitoring in Intensive Care
Read more about the importance of appropriate drug concentrations in antibiotic therapies to and help prevent over- and underdosing. This approach also reduces the risk of new multidrug resistant strains emerging and helps to provide the most effective drug dose to the patient.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 0.5–2.2 mg/l |
| Upper Limit of Quantification | up to 60–400 mg/l |
| Intraassay | CV = 0.5–1.6 % |
| Interassay | CV = 2.5–5.5 % |
| Recovery | 91–104 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 13 min (Group 1), 5 min (Group 2) |
| Injection Volume | 5 μl (Group 1), 10 μl (Group 2) |
| Flow Rate | 1 ml/min |
| Column Temperature | 22 °C |
| Gradient | binary (Group 1) |
| Wavelengths | 0–5.9 min 290 nm, then 210 nm (Group 1) |
| Additional Info | A binary HPLC gradient system with coolable injector, coolable column oven and UV-detector is required. |
| Information | Autosampler temperature: 10 °C |
| Parameters | Ampicillin, Cefepime, Ceftazidime, Linezolid, Meropenem, Piperacillin, Sulbactam, Tazobactam |
-
 3PLUS1® Multilevel Plasma Calibrator Set AntibioticsOrder no.: 61028Antibiotics in Serum/Plasma – HPLC
3PLUS1® Multilevel Plasma Calibrator Set AntibioticsOrder no.: 61028Antibiotics in Serum/Plasma – HPLC
-
 3PLUS1® Multilevel Plasma Calibrator Set AntibioticsOrder no.: 61028Antibiotics in Serum/Plasma – HPLC
3PLUS1® Multilevel Plasma Calibrator Set AntibioticsOrder no.: 61028Antibiotics in Serum/Plasma – HPLC