Parameter Set Benzodiazepines 1 - LC-MS/MS
Encompasses 13 analytes
3PLUS1® Multilevel Calibrator Set available
Part of the MassTox® TDM Series A
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
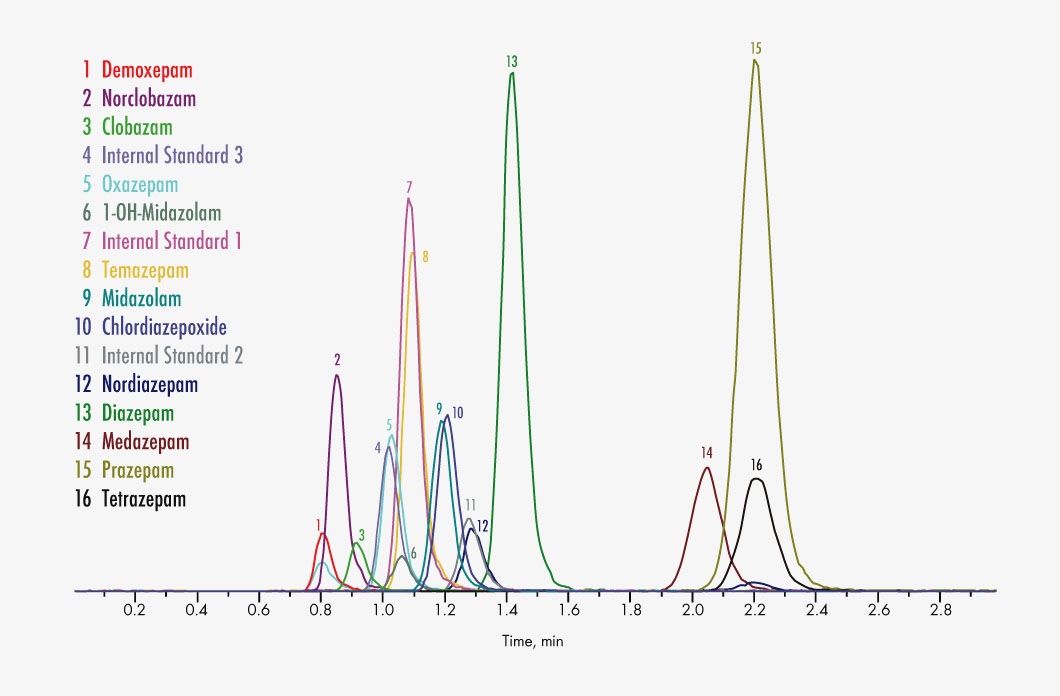

Chlordiazepoxide
Clobazam
Norclobazam
Demoxepam
Diazepam
Nordiazepam
Medazepam
Midazolam
1-OH-Midazolam
Oxazepam
Prazepam
Temazepam
Tetrazepam
Clinical relevance
Benzodiazepines (BZD) are a group of psychoactive drugs with similar structures that are classified as sedatives. By virtue of their high lipophilicity they penetrate the blood-brain barrier easily and, after being resorbed, quickly reach the central nervous system, where they enhance the impact of the inhibiting neurotransmitter GABA. Besides anxiolytic and sedative effects, BZD also have anti-aggressive, hypnotic, muscle relaxant and anticonvulsive properties. The predominance of the individual effects varies with the special structures of the benzodiazepines and the administered dose. BZD in blood are present mostly bound nonspecifically to plasma proteins. They are metabolised mainly by the liver. The resulting metabolites are mostly pharmacologically active themselves, and in some cases they represent the actual active substance, e.g. nordiazem being metabolised from diazepam or clorazepate. The plasma elimination times are highly variable: from a few hours (midazolam) to several days (clobazam). The metabolites frequently have longer half-lives than the parent substance, so an enrichment of therapeutically effective substances in the blood can occur.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 3 – 40 µg/l |
| Upper Limit of Quantification | up to 1000–5050 μg/l |
| Intraassay | CV = 2 – 5 % |
| Interassay | CV = 2 – 7 % |
| Recovery | 87 – 107 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 3 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Isocratic (25 % Mobile Phase 1; 75 % Mobile Phase 2) |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | 1-OH-Midazolam, Chlordiazepoxide, Clobazam, Demoxepam, Diazepam, Medazepam, Midazolam, Norclobazam, Nordiazepam, Oxazepam, Prazepam, Temazepam, Tetrazepam |
-
 3PLUS1® Multilevel Plasma Calibrator Set Benzodiazepines 1Order no.: 92030MassTox® TDM Series A Benzodiazepines 1 in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Benzodiazepines 1Order no.: 92030MassTox® TDM Series A Benzodiazepines 1 in Serum/Plasma – LC-MS/MS -
 Internal Standard Mix BenzodiazepinesOrder no.: 92346Component of the Parameter Sets Benzodiazepines 1 and 2, available separately
Internal Standard Mix BenzodiazepinesOrder no.: 92346Component of the Parameter Sets Benzodiazepines 1 and 2, available separately
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Benzodiazepines 1Order no.: 92020Tuning Mix for the Parameter Set Benzodiazepines 1 - LC-MS/MS
Tuning Mix Benzodiazepines 1Order no.: 92020Tuning Mix for the Parameter Set Benzodiazepines 1 - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 3PLUS1® Multilevel Plasma Calibrator Set Benzodiazepines 1Order no.: 92030MassTox® TDM Series A Benzodiazepines 1 in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Benzodiazepines 1Order no.: 92030MassTox® TDM Series A Benzodiazepines 1 in Serum/Plasma – LC-MS/MS


Chlordiazepoxide
Clobazam
Norclobazam
Demoxepam
Diazepam
Nordiazepam
Medazepam
Midazolam
1-OH-Midazolam
Oxazepam
Prazepam
Temazepam
Tetrazepam
Clinical relevance
Benzodiazepines (BZD) are a group of psychoactive drugs with similar structures that are classified as sedatives. By virtue of their high lipophilicity they penetrate the blood-brain barrier easily and, after being resorbed, quickly reach the central nervous system, where they enhance the impact of the inhibiting neurotransmitter GABA. Besides anxiolytic and sedative effects, BZD also have anti-aggressive, hypnotic, muscle relaxant and anticonvulsive properties. The predominance of the individual effects varies with the special structures of the benzodiazepines and the administered dose. BZD in blood are present mostly bound nonspecifically to plasma proteins. They are metabolised mainly by the liver. The resulting metabolites are mostly pharmacologically active themselves, and in some cases they represent the actual active substance, e.g. nordiazem being metabolised from diazepam or clorazepate. The plasma elimination times are highly variable: from a few hours (midazolam) to several days (clobazam). The metabolites frequently have longer half-lives than the parent substance, so an enrichment of therapeutically effective substances in the blood can occur.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 3 – 40 µg/l |
| Upper Limit of Quantification | up to 1000–5050 μg/l |
| Intraassay | CV = 2 – 5 % |
| Interassay | CV = 2 – 7 % |
| Recovery | 87 – 107 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 3 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Isocratic (25 % Mobile Phase 1; 75 % Mobile Phase 2) |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | 1-OH-Midazolam, Chlordiazepoxide, Clobazam, Demoxepam, Diazepam, Medazepam, Midazolam, Norclobazam, Nordiazepam, Oxazepam, Prazepam, Temazepam, Tetrazepam |
-
 3PLUS1® Multilevel Plasma Calibrator Set Benzodiazepines 1Order no.: 92030MassTox® TDM Series A Benzodiazepines 1 in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Benzodiazepines 1Order no.: 92030MassTox® TDM Series A Benzodiazepines 1 in Serum/Plasma – LC-MS/MS -
 Internal Standard Mix BenzodiazepinesOrder no.: 92346Component of the Parameter Sets Benzodiazepines 1 and 2, available separately
Internal Standard Mix BenzodiazepinesOrder no.: 92346Component of the Parameter Sets Benzodiazepines 1 and 2, available separately
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Benzodiazepines 1Order no.: 92020Tuning Mix for the Parameter Set Benzodiazepines 1 - LC-MS/MS
Tuning Mix Benzodiazepines 1Order no.: 92020Tuning Mix for the Parameter Set Benzodiazepines 1 - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 3PLUS1® Multilevel Plasma Calibrator Set Benzodiazepines 1Order no.: 92030MassTox® TDM Series A Benzodiazepines 1 in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Benzodiazepines 1Order no.: 92030MassTox® TDM Series A Benzodiazepines 1 in Serum/Plasma – LC-MS/MS