Parameter Set Antiarrhythmic Drugs - LC-MS/MS
Encompasses 25 analytes
3PLUS1® Multilevel Calibrator Set available
Part of the MassTox® TDM Series A
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746



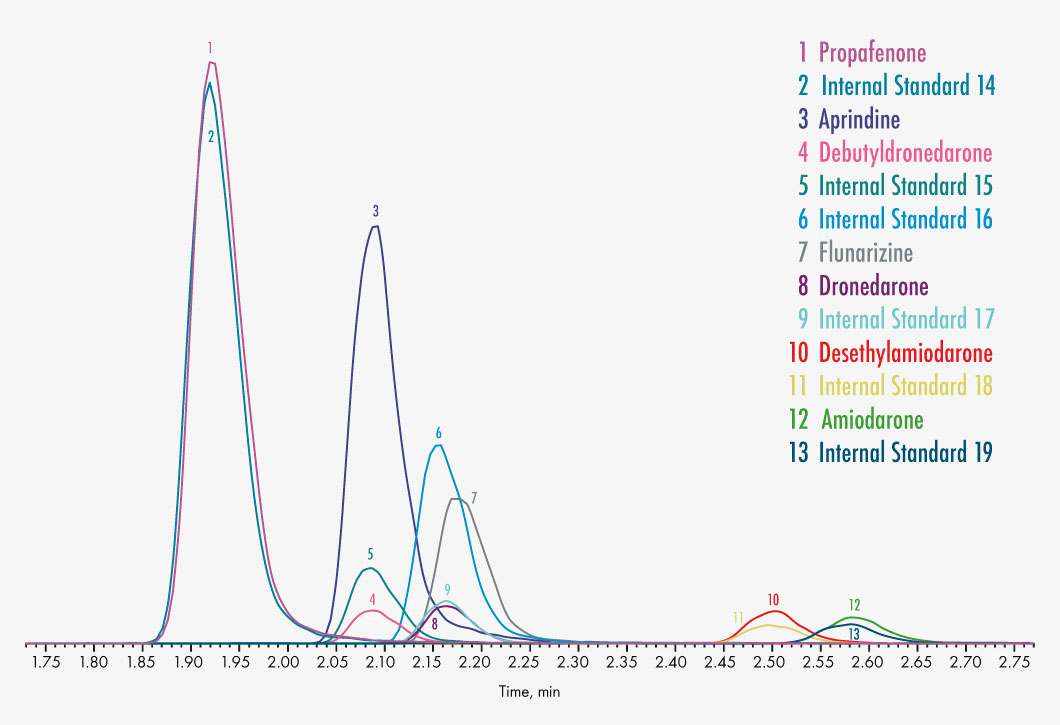

Acebutolol
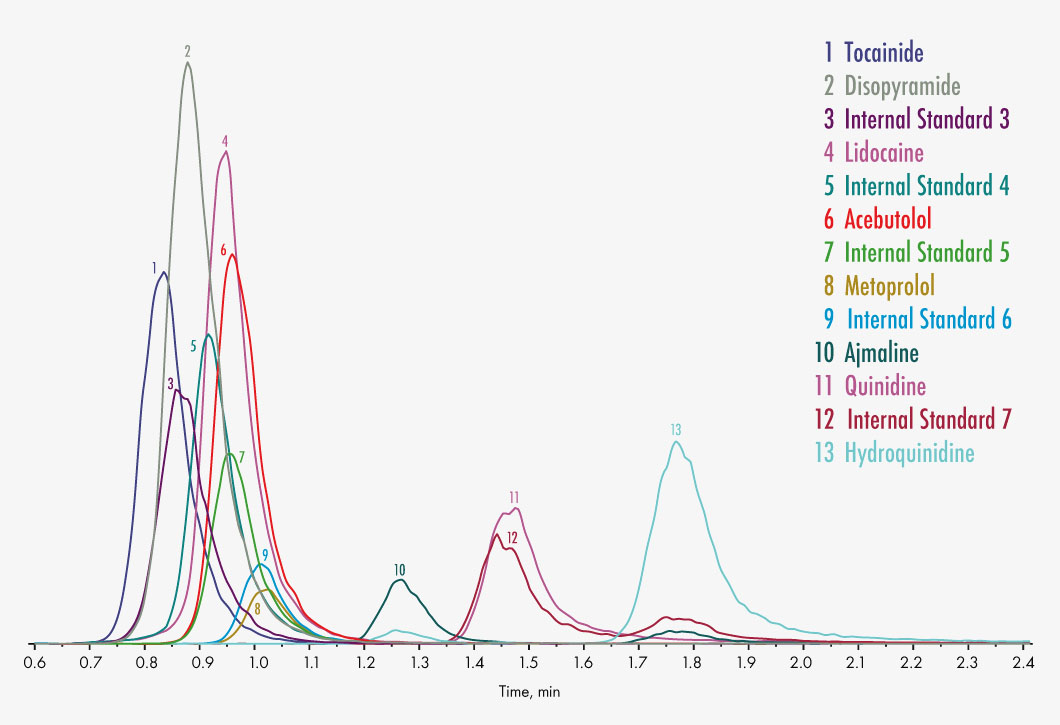
Ajmaline
Amiodarone
Desethylamiodarone
Aprindine
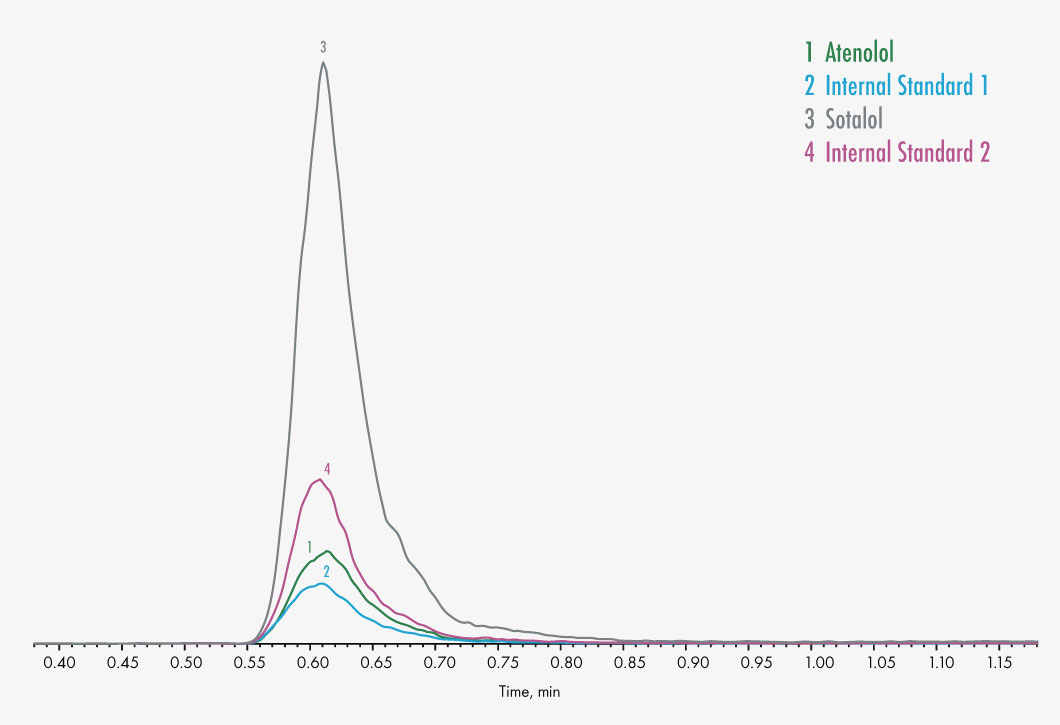
Atenolol
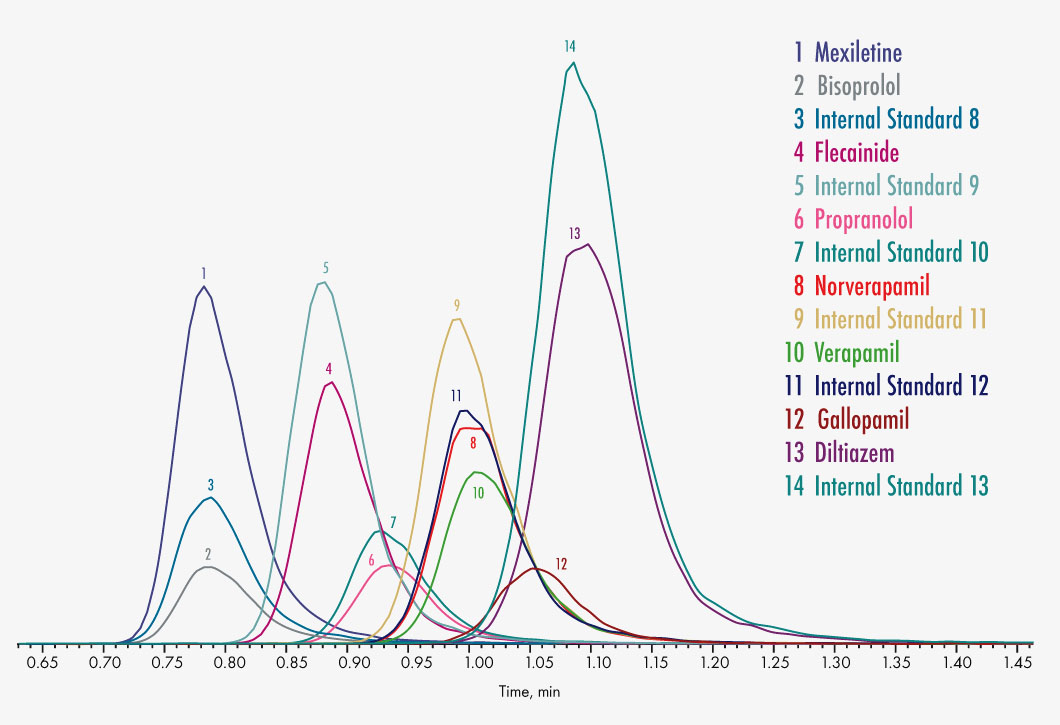
Bisoprolol
Diltiazem
Disopyramide
Dronedarone
Debutyldronedarone
Flecainide
Flunarizine
Gallopamil
Lidocaine
Metoprolol
Mexiletine
Propafenone
Propranolol
Quinidine
Hydroquinidine
Sotalol
Tocainide
Verapamil
Norverapamil
Clinical relevance
Antiarrhythmic drugs are used for the treatment of cardiac arrhythmia to reduce their frequency or intensity. The severity of ventricular and supraventricular rhythm disturbances ranges from harmless extra beats through to complex multiple blows and life-threatening tachycardia. By using antiarrhythmic agents, it is possible to restore a normal electrical cardiac activity. Since antiarrhythmic drugs are an inhomogeneous group, in their chemistry and mechanisms of action, various side effects and drug interactions have to be considered. Their use should be monitored by ECG monitoring, electrolyte and plasma level determinations, in particular when starting new drug regimens. As each antiarrhythmic drug possesses a pro-arrhythmic potential, it also can cause cardiac arrhythmia. Furthermore, there are drug-drug interactions between the different antiarrhythmics, and therefore, combinations should be taken with extreme caution as well as with due attention to the side-effects and interaction profiles. For these reasons cardiac arrhythmia are often only treated with medication when they are very dangerous or associated with a high burden for the patient.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 1.5 – 80 µg/l |
| Upper Limit of Quantification | up to 30000 µg/l |
| Intraassay | CV = 2 – 9 % |
| Interassay | CV = 3 – 8.5 % |
| Recovery | 87 – 109 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 1.2 – 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Group 1 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Parameters | Acebutolol, Ajmaline, Amiodarone, Aprindine, Atenolol, Bisoprolol, Debutyldronedarone, Desethylamiodarone, Diltiazem, Disopyramide, Dronedarone, Flecainide, Flunarizine, Gallopamil, Hydroquinidine, Lidocaine, Metoprolol, Mexiletine, Norverapamil, Propafenone, Propranolol, Quinidine, Sotalol, Tocainide, Verapamil |
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiarrhythmic DrugsOrder no.: 92052MassTox® TDM Series A Antiarrhythmic Drugs in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antiarrhythmic DrugsOrder no.: 92052MassTox® TDM Series A Antiarrhythmic Drugs in Serum/Plasma – LC-MS/MS -
 Internal Standard Set Antiarrhythmic DrugsOrder no.: 92746Component of the Parameter Set Antiarrhythmic Drugs, available separately
Internal Standard Set Antiarrhythmic DrugsOrder no.: 92746Component of the Parameter Set Antiarrhythmic Drugs, available separately
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Antiarrhythmic DrugsOrder no.: 92041Tuning Mix for the Parameter Set Antiarrhythmic Drugs - LC-MS/MS
Tuning Mix Antiarrhythmic DrugsOrder no.: 92041Tuning Mix for the Parameter Set Antiarrhythmic Drugs - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiarrhythmic DrugsOrder no.: 92052MassTox® TDM Series A Antiarrhythmic Drugs in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antiarrhythmic DrugsOrder no.: 92052MassTox® TDM Series A Antiarrhythmic Drugs in Serum/Plasma – LC-MS/MS








Acebutolol
Ajmaline
Amiodarone
Desethylamiodarone
Aprindine
Atenolol
Bisoprolol
Diltiazem
Disopyramide
Dronedarone
Debutyldronedarone
Flecainide
Flunarizine
Gallopamil
Lidocaine
Metoprolol
Mexiletine
Propafenone
Propranolol
Quinidine
Hydroquinidine
Sotalol
Tocainide
Verapamil
Norverapamil
Clinical relevance
Antiarrhythmic drugs are used for the treatment of cardiac arrhythmia to reduce their frequency or intensity. The severity of ventricular and supraventricular rhythm disturbances ranges from harmless extra beats through to complex multiple blows and life-threatening tachycardia. By using antiarrhythmic agents, it is possible to restore a normal electrical cardiac activity. Since antiarrhythmic drugs are an inhomogeneous group, in their chemistry and mechanisms of action, various side effects and drug interactions have to be considered. Their use should be monitored by ECG monitoring, electrolyte and plasma level determinations, in particular when starting new drug regimens. As each antiarrhythmic drug possesses a pro-arrhythmic potential, it also can cause cardiac arrhythmia. Furthermore, there are drug-drug interactions between the different antiarrhythmics, and therefore, combinations should be taken with extreme caution as well as with due attention to the side-effects and interaction profiles. For these reasons cardiac arrhythmia are often only treated with medication when they are very dangerous or associated with a high burden for the patient.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 1.5 – 80 µg/l |
| Upper Limit of Quantification | up to 30000 µg/l |
| Intraassay | CV = 2 – 9 % |
| Interassay | CV = 3 – 8.5 % |
| Recovery | 87 – 109 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 1.2 – 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Group 1 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Parameters | Acebutolol, Ajmaline, Amiodarone, Aprindine, Atenolol, Bisoprolol, Debutyldronedarone, Desethylamiodarone, Diltiazem, Disopyramide, Dronedarone, Flecainide, Flunarizine, Gallopamil, Hydroquinidine, Lidocaine, Metoprolol, Mexiletine, Norverapamil, Propafenone, Propranolol, Quinidine, Sotalol, Tocainide, Verapamil |
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiarrhythmic DrugsOrder no.: 92052MassTox® TDM Series A Antiarrhythmic Drugs in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antiarrhythmic DrugsOrder no.: 92052MassTox® TDM Series A Antiarrhythmic Drugs in Serum/Plasma – LC-MS/MS -
 Internal Standard Set Antiarrhythmic DrugsOrder no.: 92746Component of the Parameter Set Antiarrhythmic Drugs, available separately
Internal Standard Set Antiarrhythmic DrugsOrder no.: 92746Component of the Parameter Set Antiarrhythmic Drugs, available separately
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Antiarrhythmic DrugsOrder no.: 92041Tuning Mix for the Parameter Set Antiarrhythmic Drugs - LC-MS/MS
Tuning Mix Antiarrhythmic DrugsOrder no.: 92041Tuning Mix for the Parameter Set Antiarrhythmic Drugs - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiarrhythmic DrugsOrder no.: 92052MassTox® TDM Series A Antiarrhythmic Drugs in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antiarrhythmic DrugsOrder no.: 92052MassTox® TDM Series A Antiarrhythmic Drugs in Serum/Plasma – LC-MS/MS