Porphyrins in Urine - HPLC
Easy sample preparation
Stable standards and controls
Assay validated according to IVDR (=> Declaration of Conformity)
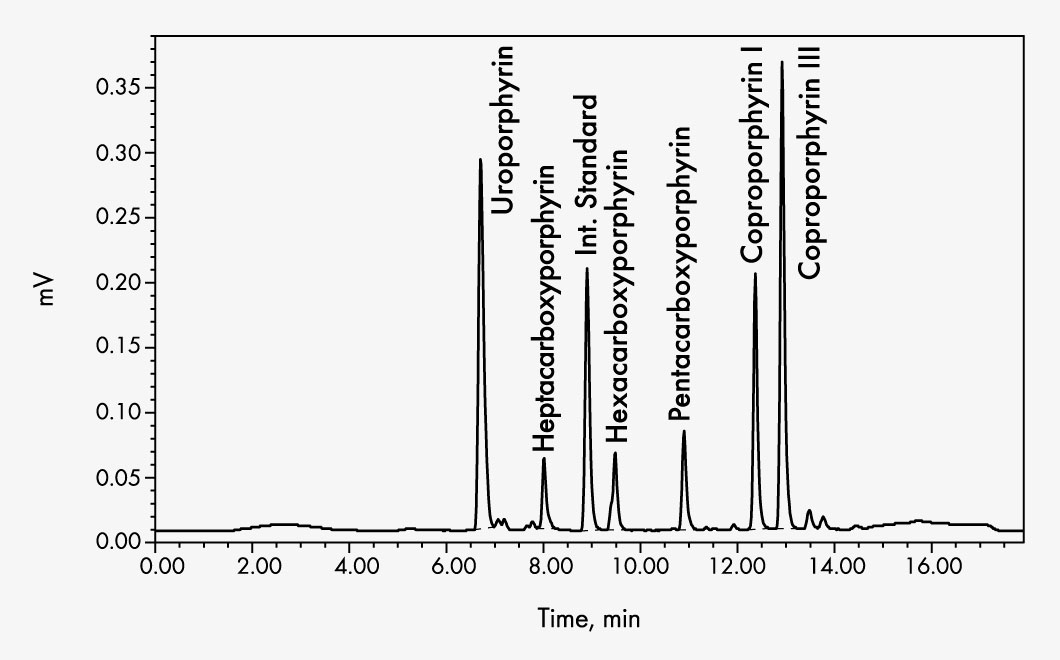

Coproporphyrin I
Coproporphyrin III
Heptacarboxyporphyrin
Hexacarboxyporphyrin
Pentacarboxyporphyrin
Uroporphyrin
Clinical relevance
This assay allows the quantitative determination of uroporphyrin, heptacarboxyporphyrin, hexacarboxyporphyrin, pentacarboxyporphyrin, coproporphyrin I and coproporphyrin III in human urine samples.
It is intended to be used for patients in whom the urinary levels of uroporphyrin, heptacarboxyporphyrin, hexacarboxyporphyrin, pentacarboxyporphyrin, coproporphyrin I and coproporphyrin III are of clinical importance, primarily as an aid to diagnosis of suspected acute intermittent porphyria, porphyria variegata, aminolevulinic acid dehydratase deficiency porphyria, hereditary coproporphyria, porphyria cutanea tarda or congenital erythropoietic porphyria and/or for their differential diagnosis.
The assay is also intended to be used as an aid to diagnosis of lead poisoning in patients in whom the urinary levels of above listed analytes are of clinical importance after suspected lead exposure (lead itself cannot be detected with this assay).
Besides, the assay is also intended to be used as an aid to diagnosis of the Dubin-Johnson syndrome in patients in whom the urinary levels of coproporphyrin I and coproporphyrin III are of clinical interest.
Product advantages
• Easy sample preparation
• Stable standards and controls
Assay characteristics
Sample preparation consists of sample stabilisation, addition of the internal standard followed by centrifugation to separate particles that may be present in the urine. An oxidation step is not necessary as the porphyrinogens oxidise spontaneously by air into the fluorescent porphyrins. The internal standard elutes between the analytes thus avoiding analytical variation and inaccuracy as well as allowing a shortening of the chromatographic run time.
Detailed performance evaluation data for this assay can be found in Appendices II and III of the instruction manual.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data for this assay can be found in Appendices II and III of the instruction manual. |
| Lower and Upper Limit of Quantitation | Uroporphyrin: 0.48 µg/l – 1400 µg/l Different systems might show different performance data. |
| Specimen | Urine |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
| Run Time | 20 min |
| Injection Volume | 25 µl |
| Flow Rate | 1.2 ml/min |
| Column Temperature | +20 to +25 °C |
| Gradient | binary |
| Wavelengths | EX 405 nm EM 620 nm |
| Additional Info | Any binary HPLC gradient system with fluorescence detector is suitable. |
| Parameters | Coproporphyrin I, Coproporphyrin III, Heptacarboxyporphyrin, Hexacarboxyporphyrin, Pentacarboxyporphyrin, Uroporphyrin |
44000 Porphyrins in Urine
For 100 tests
| Order No. | Kit components (Quantities in kit) |
| 44001 | Mobile Phase A, 1000 ml (2x) |
| 44002 | Mobile Phase B, 1000 ml (2x) |
| 44003 | Porphyrins Urine Calibration Standard (lyoph.), 5 x 2 ml (1x) |
| 44004 | Internal Standard, 5 ml (1x) |
| 44005 | Stabilisation Reagent, 5 ml (1x) |
| 44006 | Priming Solution, 5 ml (1x) |
| 33005 | Reaction Vials, amber coloured (light protection), 100 pcs. (1x) |
| Order No. | Required components (not included in the kit) |
| 44100 | HPLC Column, equilibrated, with test chromatogram, 1 pc. |
| 0145 | Porphyrins Urine Control, Level I, 5 x 2 ml |
| 0146 | Porphyrins Urine Control, Level II, 5 x 2 ml |
| 18044 | Precolumn Cartridge 4/10, 1 pcs. |
| Order No. | General lab equipment (non-CE/IVD products) |
| 33005 | Reaction Vials, amber coloured (light protection), 100 pcs. |
| 15010 | PEEK Prefilter Housing, 1 pc. |
| 15009 | PEEK-encased Prefilter, 5 µm, 5 pcs. |
| 18001 | Precolumn Cartridge Holder 4/10, 1 pc. |


Coproporphyrin I
Coproporphyrin III
Heptacarboxyporphyrin
Hexacarboxyporphyrin
Pentacarboxyporphyrin
Uroporphyrin
Clinical relevance
This assay allows the quantitative determination of uroporphyrin, heptacarboxyporphyrin, hexacarboxyporphyrin, pentacarboxyporphyrin, coproporphyrin I and coproporphyrin III in human urine samples.
It is intended to be used for patients in whom the urinary levels of uroporphyrin, heptacarboxyporphyrin, hexacarboxyporphyrin, pentacarboxyporphyrin, coproporphyrin I and coproporphyrin III are of clinical importance, primarily as an aid to diagnosis of suspected acute intermittent porphyria, porphyria variegata, aminolevulinic acid dehydratase deficiency porphyria, hereditary coproporphyria, porphyria cutanea tarda or congenital erythropoietic porphyria and/or for their differential diagnosis.
The assay is also intended to be used as an aid to diagnosis of lead poisoning in patients in whom the urinary levels of above listed analytes are of clinical importance after suspected lead exposure (lead itself cannot be detected with this assay).
Besides, the assay is also intended to be used as an aid to diagnosis of the Dubin-Johnson syndrome in patients in whom the urinary levels of coproporphyrin I and coproporphyrin III are of clinical interest.
Product advantages
• Easy sample preparation
• Stable standards and controls
Assay characteristics
Sample preparation consists of sample stabilisation, addition of the internal standard followed by centrifugation to separate particles that may be present in the urine. An oxidation step is not necessary as the porphyrinogens oxidise spontaneously by air into the fluorescent porphyrins. The internal standard elutes between the analytes thus avoiding analytical variation and inaccuracy as well as allowing a shortening of the chromatographic run time.
Detailed performance evaluation data for this assay can be found in Appendices II and III of the instruction manual.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data for this assay can be found in Appendices II and III of the instruction manual. |
| Lower and Upper Limit of Quantitation | Uroporphyrin: 0.48 µg/l – 1400 µg/l Different systems might show different performance data. |
| Specimen | Urine |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
| Run Time | 20 min |
| Injection Volume | 25 µl |
| Flow Rate | 1.2 ml/min |
| Column Temperature | +20 to +25 °C |
| Gradient | binary |
| Wavelengths | EX 405 nm EM 620 nm |
| Additional Info | Any binary HPLC gradient system with fluorescence detector is suitable. |
| Parameters | Coproporphyrin I, Coproporphyrin III, Heptacarboxyporphyrin, Hexacarboxyporphyrin, Pentacarboxyporphyrin, Uroporphyrin |
44000 Porphyrins in Urine
For 100 tests
| Order No. | Kit components (Quantities in kit) |
| 44001 | Mobile Phase A, 1000 ml (2x) |
| 44002 | Mobile Phase B, 1000 ml (2x) |
| 44003 | Porphyrins Urine Calibration Standard (lyoph.), 5 x 2 ml (1x) |
| 44004 | Internal Standard, 5 ml (1x) |
| 44005 | Stabilisation Reagent, 5 ml (1x) |
| 44006 | Priming Solution, 5 ml (1x) |
| 33005 | Reaction Vials, amber coloured (light protection), 100 pcs. (1x) |
| Order No. | Required components (not included in the kit) |
| 44100 | HPLC Column, equilibrated, with test chromatogram, 1 pc. |
| 0145 | Porphyrins Urine Control, Level I, 5 x 2 ml |
| 0146 | Porphyrins Urine Control, Level II, 5 x 2 ml |
| 18044 | Precolumn Cartridge 4/10, 1 pcs. |
| Order No. | General lab equipment (non-CE/IVD products) |
| 33005 | Reaction Vials, amber coloured (light protection), 100 pcs. |
| 15010 | PEEK Prefilter Housing, 1 pc. |
| 15009 | PEEK-encased Prefilter, 5 µm, 5 pcs. |
| 18001 | Precolumn Cartridge Holder 4/10, 1 pc. |