Parameter Set Antiepileptic Drugs XT2 All-in-One Method - LC-MS/MS
Updated parameter menu including cenobamate
Each analyte covered by its own internal standard
Determination of all analytes in a single run
3PLUS1® Multilevel Calibrator Set and MassCheck® controls
Part of the continuously extended MassTox® TDM Series A
Validated according to IVDR (=> Declaration of Conformity)

Clinical relevance
Epileptic seizures are the result of synchronous discharges of neuron groups in the brain that can lead to sudden and involuntary stereotypical behavioural or sensory disorders. Numerous types of seizures have been described, each of which requires specialised therapy. The probability of them occurring depends on a number of factors. In addition to genetic predisposition, also exogenous factors are relevant, such as accident, thrombosis, tumours or meningitis. Therapy with anticonvulsive medication (antiepileptics) leads to a reduction in seizures and sometimes even a complete elimination of seizures in most treated patients. The precondition for the antiepileptics to have a therapeutic effect is usually patient compliance, which means regular use of the medication. Thus, monitoring of the blood levels is essential.
Theophylline, which can also be determined with this assay, is primarily used to treat asthma and chronic obstructive pulmonary disease (COPD). An overdose of this substance can lead to seizures.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
Detailed performance evaluation data can be found in Appendices II and III of the instruction manual.
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data can be found in Appendices II and III of the instruction manual. |
| Lower and Upper Limit of Quantitation | Brivaracetam: 0.11 mg/L – 9.00 mg/L For the values of all analytes please refer to the instruction manual. Different systems might show different performance data. |
| Specimen | Serum/Plasma |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
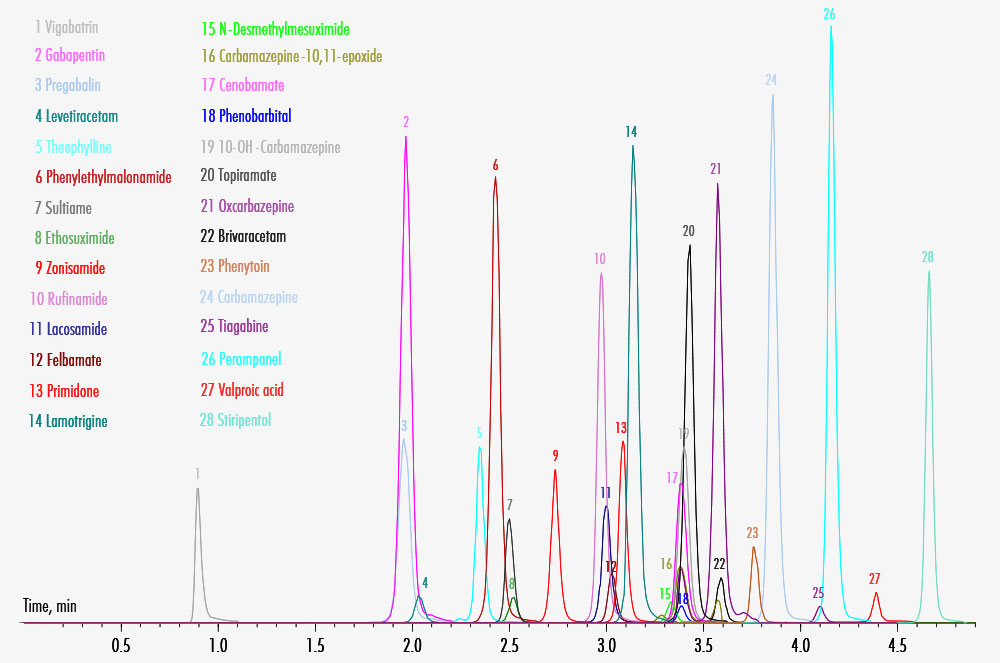
| Run Time | 4.9 min |
| Injection Volume | ≤ 10 µL |
| Gradient | binary |
| Additional Info | Autosampler cooling recommended |
| Ionisation | ESI positive and negative |
| MS/MS Mode | MRM |
| Additional Info | This method further includes 28 internal standards, which are not shown in the chromatogram for clarity. To avoid ESI switching for ethosuximide, phenobarbital and valproic acid, the analysis can be performed separately in ESI negative mode. |
| Parameters | 10-OH-Carbamazepine, Brivaracetam, Carbamazepine, Carbamazepine-10,11-epoxide, Cenobamate, Ethosuximide, Felbamate, Gabapentin, Lacosamide, Lamotrigine, Levetiracetam, N-Desmethylmesuximide, Oxcarbamazepine, Perampanel, Phenobarbital, Phenylethylmalonamide (PEMA), Phenytoin, Pregabalin, Primidone, Rufinamide, Stiripentol, Sultiame, Theophylline, Tiagabine, Topiramate, Valproic Acid, Vigabatrin, Zonisamide |
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs XT2Order no.: 92025-XT2MassTox® TDM Series A
3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs XT2Order no.: 92025-XT2MassTox® TDM Series A -
 MassCheck® Antiepileptic Drugs XT2 Plasma ControlsOrder no.: 0249-XT2; 0250-XT2; 0251-XT2MassTox® TDM Series A
MassCheck® Antiepileptic Drugs XT2 Plasma ControlsOrder no.: 0249-XT2; 0250-XT2; 0251-XT2MassTox® TDM Series A
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs XT2Order no.: 92025-XT2MassTox® TDM Series A
3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs XT2Order no.: 92025-XT2MassTox® TDM Series A
-
 MassCheck® Antiepileptic Drugs XT2 Plasma ControlsOrder no.: 0249-XT2; 0250-XT2; 0251-XT2MassTox® TDM Series A
MassCheck® Antiepileptic Drugs XT2 Plasma ControlsOrder no.: 0249-XT2; 0250-XT2; 0251-XT2MassTox® TDM Series A


Clinical relevance
Epileptic seizures are the result of synchronous discharges of neuron groups in the brain that can lead to sudden and involuntary stereotypical behavioural or sensory disorders. Numerous types of seizures have been described, each of which requires specialised therapy. The probability of them occurring depends on a number of factors. In addition to genetic predisposition, also exogenous factors are relevant, such as accident, thrombosis, tumours or meningitis. Therapy with anticonvulsive medication (antiepileptics) leads to a reduction in seizures and sometimes even a complete elimination of seizures in most treated patients. The precondition for the antiepileptics to have a therapeutic effect is usually patient compliance, which means regular use of the medication. Thus, monitoring of the blood levels is essential.
Theophylline, which can also be determined with this assay, is primarily used to treat asthma and chronic obstructive pulmonary disease (COPD). An overdose of this substance can lead to seizures.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
Detailed performance evaluation data can be found in Appendices II and III of the instruction manual.
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data can be found in Appendices II and III of the instruction manual. |
| Lower and Upper Limit of Quantitation | Brivaracetam: 0.11 mg/L – 9.00 mg/L For the values of all analytes please refer to the instruction manual. Different systems might show different performance data. |
| Specimen | Serum/Plasma |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
| Run Time | 4.9 min |
| Injection Volume | ≤ 10 µL |
| Gradient | binary |
| Additional Info | Autosampler cooling recommended |
| Ionisation | ESI positive and negative |
| MS/MS Mode | MRM |
| Additional Info | This method further includes 28 internal standards, which are not shown in the chromatogram for clarity. To avoid ESI switching for ethosuximide, phenobarbital and valproic acid, the analysis can be performed separately in ESI negative mode. |
| Parameters | 10-OH-Carbamazepine, Brivaracetam, Carbamazepine, Carbamazepine-10,11-epoxide, Cenobamate, Ethosuximide, Felbamate, Gabapentin, Lacosamide, Lamotrigine, Levetiracetam, N-Desmethylmesuximide, Oxcarbamazepine, Perampanel, Phenobarbital, Phenylethylmalonamide (PEMA), Phenytoin, Pregabalin, Primidone, Rufinamide, Stiripentol, Sultiame, Theophylline, Tiagabine, Topiramate, Valproic Acid, Vigabatrin, Zonisamide |
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs XT2Order no.: 92025-XT2MassTox® TDM Series A
3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs XT2Order no.: 92025-XT2MassTox® TDM Series A -
 MassCheck® Antiepileptic Drugs XT2 Plasma ControlsOrder no.: 0249-XT2; 0250-XT2; 0251-XT2MassTox® TDM Series A
MassCheck® Antiepileptic Drugs XT2 Plasma ControlsOrder no.: 0249-XT2; 0250-XT2; 0251-XT2MassTox® TDM Series A
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs XT2Order no.: 92025-XT2MassTox® TDM Series A
3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs XT2Order no.: 92025-XT2MassTox® TDM Series A
-
 MassCheck® Antiepileptic Drugs XT2 Plasma ControlsOrder no.: 0249-XT2; 0250-XT2; 0251-XT2MassTox® TDM Series A
MassCheck® Antiepileptic Drugs XT2 Plasma ControlsOrder no.: 0249-XT2; 0250-XT2; 0251-XT2MassTox® TDM Series A