Parameter Set Anti-HIV Drugs - LC-MS/MS
Encompasses 17 analytes
6PLUS1® Multilevel Calibrator Set available
Part of the MassTox® TDM Series A
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
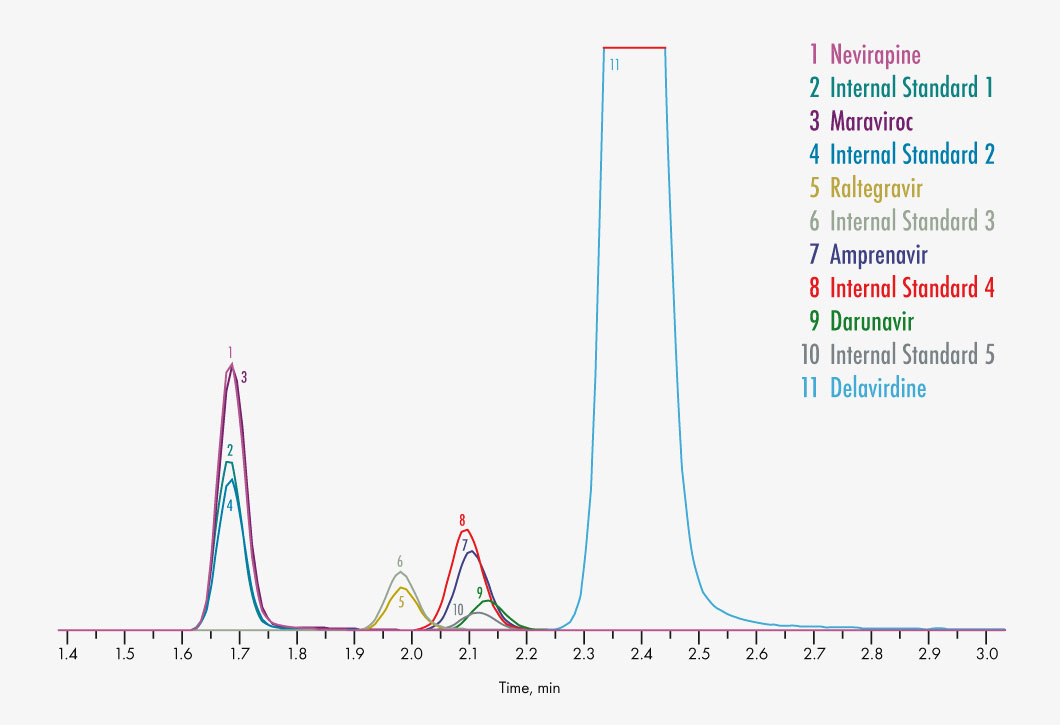

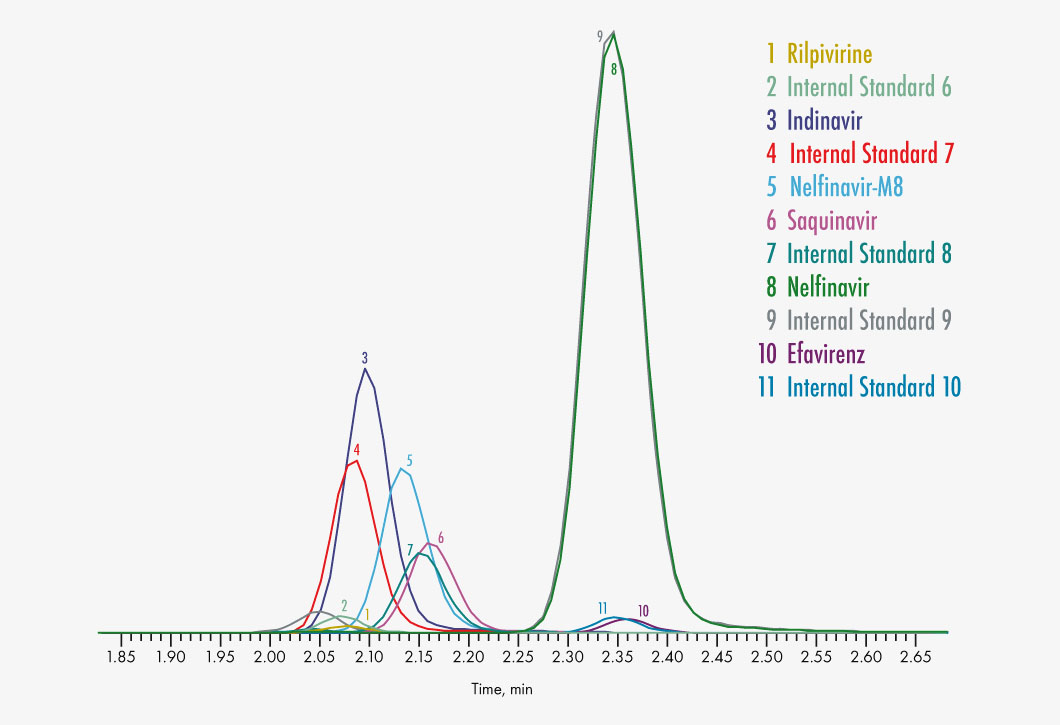

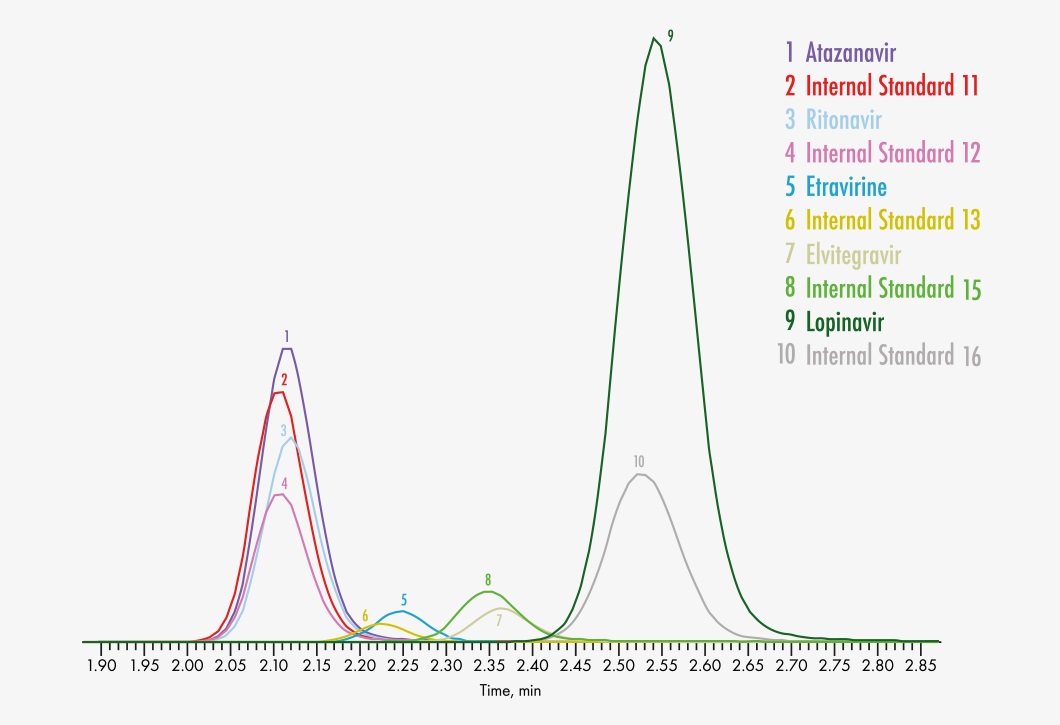

Amprenavir
Atazanavir
Darunavir
Delavirdine
Efavirenz
Elvitegravir
Etravirine
Indinavir
Lopinavir
Maraviroc
Nelfinavir
Nelfinavir-M8
Nevirapine
Raltegravir
Rilpivirine
Ritonavir
Saquinavir
Clinical relevance
According to estimates from UNAIDS there are about 36.9 million people worldwide living with HIV, of which approximately 3 million are children under the age of 15 years. Even if the number of globally registered new infections is decreasing, it was still at approx. 2 million in 2014. The currently available drugs, which are usually prescribed as part of HAART (highly active antiretroviral therapy), interfere with or prevent the development of the virus at different stages of reproduction or cell infection.
Regular monitoring of drug concentration is very important, especially in HIV therapy. Sufficiently high levels of the administered antiretroviral drugs in plasma are a key factor in the success of the treatment. Individual plasma levels can fluctuate significantly and low levels can affect the success of the treatment. On the other hand high concentrations can cause major side effects, such as disorders of the central nervous system, liver toxicity, gastrointestinal disorders or renal toxicity. Therefore the measurement of drug concentrations in serum or plasma is essential for optimising the therapy.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 1.0 – 65 µg/l |
| Upper Limit of Quantification | up to 168 mg/l |
| Intraassay | CV= 1.0 – 6.7 % |
| Interassay | CV= 1.5 – 8.1 % |
| Recovery | 88 – 111 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 3.0 – 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Group 1 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | Amprenavir, Atazanavir, Darunavir, Efavirenz, Elvitegravir, Etravirine, Indinavir, Lopinavir, Maraviroc, Nelfinavir, Nelfinavir-M8, Raltegravir, Ritonavir, Saquinavir |
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS -
 Internal Standard Mix Anti-HIV DrugsOrder no.: 92844Component of the Parameter Set Anti-HIV Drugs, available separately
Internal Standard Mix Anti-HIV DrugsOrder no.: 92844Component of the Parameter Set Anti-HIV Drugs, available separately
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Anti-HIV DrugsOrder no.: 92042Tuning Mix for the Parameter Set Anti-HIV Drugs - LC-MS/MS
Tuning Mix Anti-HIV DrugsOrder no.: 92042Tuning Mix for the Parameter Set Anti-HIV Drugs - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS






Amprenavir
Atazanavir
Darunavir
Delavirdine
Efavirenz
Elvitegravir
Etravirine
Indinavir
Lopinavir
Maraviroc
Nelfinavir
Nelfinavir-M8
Nevirapine
Raltegravir
Rilpivirine
Ritonavir
Saquinavir
Clinical relevance
According to estimates from UNAIDS there are about 36.9 million people worldwide living with HIV, of which approximately 3 million are children under the age of 15 years. Even if the number of globally registered new infections is decreasing, it was still at approx. 2 million in 2014. The currently available drugs, which are usually prescribed as part of HAART (highly active antiretroviral therapy), interfere with or prevent the development of the virus at different stages of reproduction or cell infection.
Regular monitoring of drug concentration is very important, especially in HIV therapy. Sufficiently high levels of the administered antiretroviral drugs in plasma are a key factor in the success of the treatment. Individual plasma levels can fluctuate significantly and low levels can affect the success of the treatment. On the other hand high concentrations can cause major side effects, such as disorders of the central nervous system, liver toxicity, gastrointestinal disorders or renal toxicity. Therefore the measurement of drug concentrations in serum or plasma is essential for optimising the therapy.
MassTox® TDM Series A
The MassTox® TDM Series A is a modular system that enables the determination of 200 analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® TDM Basic Kit A
• Specific MassTox® TDM Parameter Set (13 different parameter sets available)
• Analytical column MassTox® TDM MasterColumn® A
![]() More information about MassTox® TDM Series A
More information about MassTox® TDM Series A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | 1.0 – 65 µg/l |
| Upper Limit of Quantification | up to 168 mg/l |
| Intraassay | CV= 1.0 – 6.7 % |
| Interassay | CV= 1.5 – 8.1 % |
| Recovery | 88 – 111 % |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 3.0 – 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Group 1 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | Amprenavir, Atazanavir, Darunavir, Efavirenz, Elvitegravir, Etravirine, Indinavir, Lopinavir, Maraviroc, Nelfinavir, Nelfinavir-M8, Raltegravir, Ritonavir, Saquinavir |
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS -
 Internal Standard Mix Anti-HIV DrugsOrder no.: 92844Component of the Parameter Set Anti-HIV Drugs, available separately
Internal Standard Mix Anti-HIV DrugsOrder no.: 92844Component of the Parameter Set Anti-HIV Drugs, available separately
-
 MassTox® TDM MasterColumn® AOrder no.: 92110
MassTox® TDM MasterColumn® AOrder no.: 92110Analytical column for MassTox® TDM Series A - LC-MS/MS
-
 Tuning Mix Anti-HIV DrugsOrder no.: 92042Tuning Mix for the Parameter Set Anti-HIV Drugs - LC-MS/MS
Tuning Mix Anti-HIV DrugsOrder no.: 92042Tuning Mix for the Parameter Set Anti-HIV Drugs - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000Part of the MassTox® TDM Series A
Modular system for therapeutic drug monitoring
Provides all components required for sample prep and all mobile phasesValidated according to IVDR
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® TDM Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS