CDT in Serum with Fast Elution - HPLC
Short analysis time
Detects established variants
Exact separation of asialo-, disialo- and trisialotransferrin < 6 min
Manual or automated sample preparation with 96 well plate
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
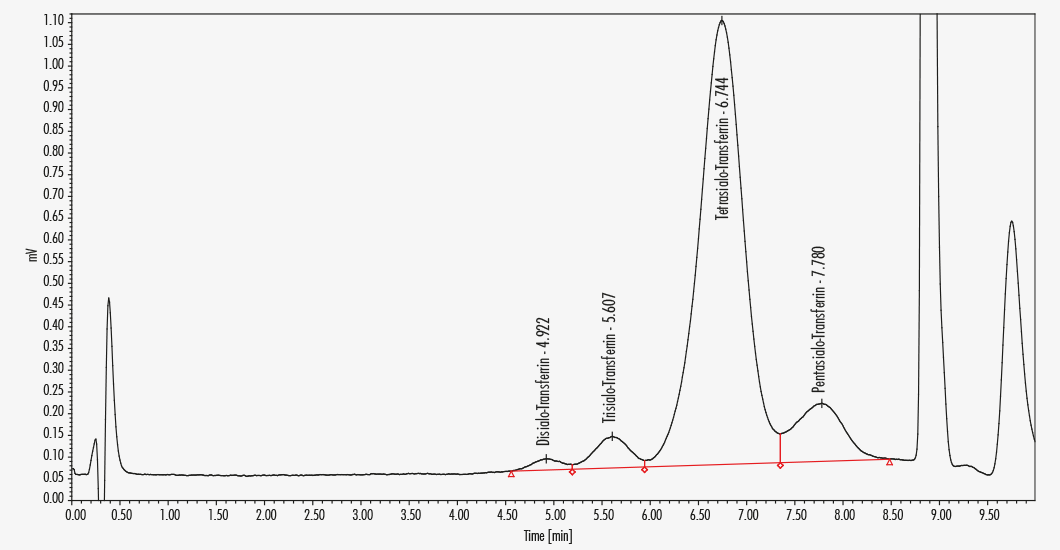

Asialotransferrin
Disialotransferrin
Pentasialotransferrin
Tetrasialotransferrin
Trisialotransferrin
Clinical relevance
Excessive alcohol consumption leads to specific changes in the glycosylation pattern of transferrin. This results in the increasing formation of asialo- and disialotransferrin in the serum, which are referred to as Carbohydrate-Deficient-Transferrins (CDT). CDT and in particular disialotransferrin is regarded as the most specific and most sensitive laboratory marker of chronic alcohol abuse. A daily intake of more than 60 g ethanol (about 0.75 l wine) over a longer period elevates CDT values significantly. These return to normal after alcohol abstinence. This makes CDT an excellent parameter for controlling withdrawal treatment, forensic assessment or in occupational medicine.
Product advantages
- Specific marker for chronic alcohol abuse
- Run time of less than 10 min
- Detection of genetic variants
- Traceable to the IFCC reference measurement procedure
This Chromsystems assay is designed for the specific and fast determination of CDT in serum with a ternary HPLC gradient system. During sample preparation, lipoproteins are removed by precipitation with simultaneous iron saturation. The chromatography enables a very good separation of asialo-Tf, disialo-Tf and trisialo-Tf in less than 6 minutes. The transferrins are separated using two mobile phases, thereafter the column is cleaned with a third mobile phase.
This method is traceable to the IFCC reference measurement procedure (IFCC-RMP) and certified accordingly. It can therefore be used to determine CDTIFCC (CDT as disialotransferrin).
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 500 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | approx. 0.5 % Disialotransferrin |
| Upper Limit of Quantification | up to at least 12 % Disialotransferrin |
| Intraassay | CV = 5.7 % |
| Interassay | CV = 6.3 % |
| Specimen | Serum |
| Sample Preparation |
* The reaction mix is prepared from Neutralisation Buffer, Stabilisation Buffer, Precipitation Reagent 1 and 2 (25 µl each per sample) |
| Run Time | 9.5 min |
| Injection Volume | 100-150 µl |
| Flow Rate | 2.5 ml/min |
| Column Temperature | +30 °C |
| Wavelengths | 460 nm |
| Additional Info | For the Chromsystems HPLC analysis of CDT FE in serum (Fast Elution) a ternary HPLC gradient system with UV detection is necessary. |
| Parameters | Asialotransferrin, Disialotransferrin, Pentasialotransferrin, Tetrasialotransferrin, Trisialotransferrin |


Asialotransferrin
Disialotransferrin
Pentasialotransferrin
Tetrasialotransferrin
Trisialotransferrin
Clinical relevance
Excessive alcohol consumption leads to specific changes in the glycosylation pattern of transferrin. This results in the increasing formation of asialo- and disialotransferrin in the serum, which are referred to as Carbohydrate-Deficient-Transferrins (CDT). CDT and in particular disialotransferrin is regarded as the most specific and most sensitive laboratory marker of chronic alcohol abuse. A daily intake of more than 60 g ethanol (about 0.75 l wine) over a longer period elevates CDT values significantly. These return to normal after alcohol abstinence. This makes CDT an excellent parameter for controlling withdrawal treatment, forensic assessment or in occupational medicine.
Product advantages
- Specific marker for chronic alcohol abuse
- Run time of less than 10 min
- Detection of genetic variants
- Traceable to the IFCC reference measurement procedure
This Chromsystems assay is designed for the specific and fast determination of CDT in serum with a ternary HPLC gradient system. During sample preparation, lipoproteins are removed by precipitation with simultaneous iron saturation. The chromatography enables a very good separation of asialo-Tf, disialo-Tf and trisialo-Tf in less than 6 minutes. The transferrins are separated using two mobile phases, thereafter the column is cleaned with a third mobile phase.
This method is traceable to the IFCC reference measurement procedure (IFCC-RMP) and certified accordingly. It can therefore be used to determine CDTIFCC (CDT as disialotransferrin).
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 500 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | approx. 0.5 % Disialotransferrin |
| Upper Limit of Quantification | up to at least 12 % Disialotransferrin |
| Intraassay | CV = 5.7 % |
| Interassay | CV = 6.3 % |
| Specimen | Serum |
| Sample Preparation |
* The reaction mix is prepared from Neutralisation Buffer, Stabilisation Buffer, Precipitation Reagent 1 and 2 (25 µl each per sample) |
| Run Time | 9.5 min |
| Injection Volume | 100-150 µl |
| Flow Rate | 2.5 ml/min |
| Column Temperature | +30 °C |
| Wavelengths | 460 nm |
| Additional Info | For the Chromsystems HPLC analysis of CDT FE in serum (Fast Elution) a ternary HPLC gradient system with UV detection is necessary. |
| Parameters | Asialotransferrin, Disialotransferrin, Pentasialotransferrin, Tetrasialotransferrin, Trisialotransferrin |