CDT in Serum - binary gradient systems - HPLC
Sample preparation: just 2 pipetting steps
Long column lifetime
Low cost per test
Reference method for CDT analyses: currently HPLC
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
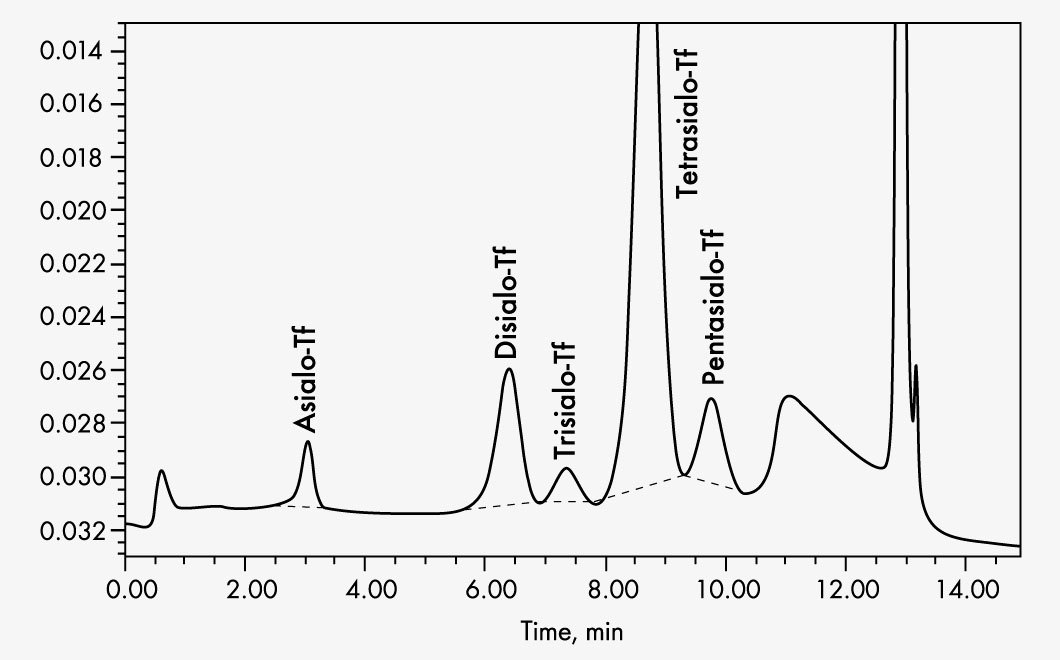

Asialotransferrin
Disialotransferrin
Pentasialotransferrin
Tetrasialotransferrin
Trisialotransferrin
Clinical relevance:
Carbohydrate deficient transferrin (CDT) is regarded as the most specific laboratory marker of chronic alcohol abuse. Unlike other biochemical parameters such as γ-GT or MCV, false positive results caused by non-alcoholic liver diseases can be excluded. A daily intake of more than 60 g ethanol (about 0.75 l wine) over a period of two weeks elevates CDT values significantly. After approx. 2 weeks of alcohol abstention, the levels normalise again. This makes CDT an excellent parameter for controlling withdrawal treatment, forensic judgements or in occupational medicine.
Product advantages:
- Specific marker of chronic alcohol abuse
- Long column lifetime
- Traceable to the IFCC reference measurement procedure (ternary method)
This Chromsystems assay is designed for the fast and reliable determination of CDT in serum using HPLC with UV detection. The results are calculated as area percent of total transferrin, and, therefore, are not affected by variations of the total transferrin concentration. The optimised chromatographic separation of the transferrin isoforms also ensures the detection of genetic variants of transferrin.
| Method of Analysis | HPLC |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | approx. 0.5 % Disialotransferrin |
| Upper Limit of Quantification | up to at least 12 % Disialotransferrin |
| Intraassay | CV ≤ 7.7 % |
| Interassay | CV ≤ 4.6 % |
| Specimen | Serum |
| Sample Preparation |
* The reaction mix is prepared from Neutralisation Buffer, Stabilisation Buffer, Precipitation Reagent 1 and 2 (25 μl each per sample) |
| Run Time | 22 min |
| Injection Volume | 200 µl |
| Flow Rate | 1.5 ml/min |
| Column Temperature | ambient (~25 °C) |
| Wavelengths | 460 nm |
| Additional Info | For the Chromsystems HPLC analysis of CDT in serum any binary HPLC gradient system with UV detection is suitable. |
| Parameters | Asialotransferrin, Disialotransferrin, Pentasialotransferrin, Tetrasialotransferrin, Trisialotransferrin |


Asialotransferrin
Disialotransferrin
Pentasialotransferrin
Tetrasialotransferrin
Trisialotransferrin
Clinical relevance:
Carbohydrate deficient transferrin (CDT) is regarded as the most specific laboratory marker of chronic alcohol abuse. Unlike other biochemical parameters such as γ-GT or MCV, false positive results caused by non-alcoholic liver diseases can be excluded. A daily intake of more than 60 g ethanol (about 0.75 l wine) over a period of two weeks elevates CDT values significantly. After approx. 2 weeks of alcohol abstention, the levels normalise again. This makes CDT an excellent parameter for controlling withdrawal treatment, forensic judgements or in occupational medicine.
Product advantages:
- Specific marker of chronic alcohol abuse
- Long column lifetime
- Traceable to the IFCC reference measurement procedure (ternary method)
This Chromsystems assay is designed for the fast and reliable determination of CDT in serum using HPLC with UV detection. The results are calculated as area percent of total transferrin, and, therefore, are not affected by variations of the total transferrin concentration. The optimised chromatographic separation of the transferrin isoforms also ensures the detection of genetic variants of transferrin.
| Method of Analysis | HPLC |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | approx. 0.5 % Disialotransferrin |
| Upper Limit of Quantification | up to at least 12 % Disialotransferrin |
| Intraassay | CV ≤ 7.7 % |
| Interassay | CV ≤ 4.6 % |
| Specimen | Serum |
| Sample Preparation |
* The reaction mix is prepared from Neutralisation Buffer, Stabilisation Buffer, Precipitation Reagent 1 and 2 (25 μl each per sample) |
| Run Time | 22 min |
| Injection Volume | 200 µl |
| Flow Rate | 1.5 ml/min |
| Column Temperature | ambient (~25 °C) |
| Wavelengths | 460 nm |
| Additional Info | For the Chromsystems HPLC analysis of CDT in serum any binary HPLC gradient system with UV detection is suitable. |
| Parameters | Asialotransferrin, Disialotransferrin, Pentasialotransferrin, Tetrasialotransferrin, Trisialotransferrin |