Mycophenolic Acid in Plasma/Serum - HPLC
Fast and easy sample preparation
Detection of physiologically active MPA
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
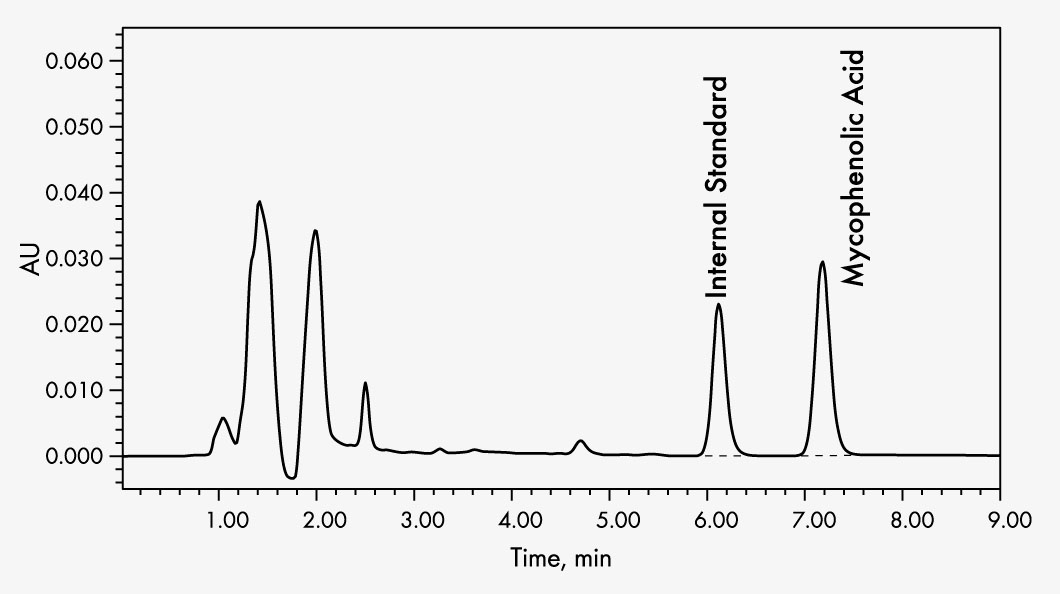

Mycophenolic Acid
Clinical relevance
Mycophenolic acid (MPA) is used as an immunosuppressant agent to prevent organ transplant rejection. It is also used to treat autoimmune disorders with chronic inflammation of the skin (psoriasis), eyes, and the digestive tract (Crohn's disease).
Currently available drugs contain mycophenolate mofetil or mycophenolate sodium, which are both completely metabolised into the active metabolite MPA. MPA is partially further metabolised into the inactive mycophenolate glucuronide (MPAG), which then can undergo deglucuronidation in the enterohepatic circulation system and is available again in the body as an active metabolite. Therapeutic monitoring of MPA is required to reach blood concentrations within the therapeutic window to achieve a favourable ratio between therapeutic effects and side effects for the patient.
The Chromsystems assay allows the quantitative detection of mycophenolic acid in human plasma or serum samples by HPLC (high performance liquid chromatography).
It is intended as a monitoring test for patients treated with mycophenolate mofetil or mycophenolate sodium as an aid to ensure drug levels are maintained within the therapeutic range.
Product advantages
• Fast and easy sample preparation
• Detection of physiologically active MPA
Assay characteristics
Sample preparation is performed by means of selective solid phase extraction, in which physiologically inactive MPAG is separated. The analyte is quantified by the inclusion of an internal standard to compensate losses during sample preparation.
Our TDM Parameter Set Mycophenolic Acid from the MassTox® TDM Series A is available as an alternative for the measurement of MPA and MPAG using LC-MS/MS.
Detailed performance evaluation data for this assay can be found in the appendices of the instruction manual.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data for this assay can be found in the appendices of the instruction manual. |
| Lower and Upper Limit of Quantitation | 0.1 mg/l – 30 mg/l |
| Specimen | Plasma/Serum |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
| Run Time | 8–9 min |
| Injection Volume | 20–40 µl |
| Flow Rate | 1 ml/min |
| Column Temperature | ambient (~ 25 °C) |
| Gradient | isocratic |
| Wavelengths | 215 nm |
| Additional Info | Any isocratic HPLC system with UV detector is suitable. |
| Parameters | Mycophenolic Acid |
-
 Mycophenolic Acid Plasma Calibration StandardOrder no.: 46003Mycophenolic Acid in Plasma/Serum - HPLC
Mycophenolic Acid Plasma Calibration StandardOrder no.: 46003Mycophenolic Acid in Plasma/Serum - HPLC
-
 HPLC Column Mycophenolic Acid in Plasma/SerumOrder no.: 46110/HRMycophenolic Acid in Plasma/Serum - HPLC
HPLC Column Mycophenolic Acid in Plasma/SerumOrder no.: 46110/HRMycophenolic Acid in Plasma/Serum - HPLC -
 Mycophenolic Acid Plasma ControlsOrder no.: 0041/0042/0043
Mycophenolic Acid Plasma ControlsOrder no.: 0041/0042/0043Mycophenolic Acid in Plasma/Serum - HPLC
-
 Mycophenolic Acid Plasma Calibration StandardOrder no.: 46003Mycophenolic Acid in Plasma/Serum - HPLC
Mycophenolic Acid Plasma Calibration StandardOrder no.: 46003Mycophenolic Acid in Plasma/Serum - HPLC
-
 Mycophenolic Acid Plasma ControlsOrder no.: 0041/0042/0043
Mycophenolic Acid Plasma ControlsOrder no.: 0041/0042/0043Mycophenolic Acid in Plasma/Serum - HPLC
-
 HPLC Column Mycophenolic Acid in Plasma/SerumOrder no.: 46110/HRMycophenolic Acid in Plasma/Serum - HPLC
HPLC Column Mycophenolic Acid in Plasma/SerumOrder no.: 46110/HRMycophenolic Acid in Plasma/Serum - HPLC


Mycophenolic Acid
Clinical relevance
Mycophenolic acid (MPA) is used as an immunosuppressant agent to prevent organ transplant rejection. It is also used to treat autoimmune disorders with chronic inflammation of the skin (psoriasis), eyes, and the digestive tract (Crohn's disease).
Currently available drugs contain mycophenolate mofetil or mycophenolate sodium, which are both completely metabolised into the active metabolite MPA. MPA is partially further metabolised into the inactive mycophenolate glucuronide (MPAG), which then can undergo deglucuronidation in the enterohepatic circulation system and is available again in the body as an active metabolite. Therapeutic monitoring of MPA is required to reach blood concentrations within the therapeutic window to achieve a favourable ratio between therapeutic effects and side effects for the patient.
The Chromsystems assay allows the quantitative detection of mycophenolic acid in human plasma or serum samples by HPLC (high performance liquid chromatography).
It is intended as a monitoring test for patients treated with mycophenolate mofetil or mycophenolate sodium as an aid to ensure drug levels are maintained within the therapeutic range.
Product advantages
• Fast and easy sample preparation
• Detection of physiologically active MPA
Assay characteristics
Sample preparation is performed by means of selective solid phase extraction, in which physiologically inactive MPAG is separated. The analyte is quantified by the inclusion of an internal standard to compensate losses during sample preparation.
Our TDM Parameter Set Mycophenolic Acid from the MassTox® TDM Series A is available as an alternative for the measurement of MPA and MPAG using LC-MS/MS.
Detailed performance evaluation data for this assay can be found in the appendices of the instruction manual.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data for this assay can be found in the appendices of the instruction manual. |
| Lower and Upper Limit of Quantitation | 0.1 mg/l – 30 mg/l |
| Specimen | Plasma/Serum |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
| Run Time | 8–9 min |
| Injection Volume | 20–40 µl |
| Flow Rate | 1 ml/min |
| Column Temperature | ambient (~ 25 °C) |
| Gradient | isocratic |
| Wavelengths | 215 nm |
| Additional Info | Any isocratic HPLC system with UV detector is suitable. |
| Parameters | Mycophenolic Acid |
-
 Mycophenolic Acid Plasma Calibration StandardOrder no.: 46003Mycophenolic Acid in Plasma/Serum - HPLC
Mycophenolic Acid Plasma Calibration StandardOrder no.: 46003Mycophenolic Acid in Plasma/Serum - HPLC
-
 HPLC Column Mycophenolic Acid in Plasma/SerumOrder no.: 46110/HRMycophenolic Acid in Plasma/Serum - HPLC
HPLC Column Mycophenolic Acid in Plasma/SerumOrder no.: 46110/HRMycophenolic Acid in Plasma/Serum - HPLC -
 Mycophenolic Acid Plasma ControlsOrder no.: 0041/0042/0043
Mycophenolic Acid Plasma ControlsOrder no.: 0041/0042/0043Mycophenolic Acid in Plasma/Serum - HPLC
-
 Mycophenolic Acid Plasma Calibration StandardOrder no.: 46003Mycophenolic Acid in Plasma/Serum - HPLC
Mycophenolic Acid Plasma Calibration StandardOrder no.: 46003Mycophenolic Acid in Plasma/Serum - HPLC
-
 Mycophenolic Acid Plasma ControlsOrder no.: 0041/0042/0043
Mycophenolic Acid Plasma ControlsOrder no.: 0041/0042/0043Mycophenolic Acid in Plasma/Serum - HPLC
-
 HPLC Column Mycophenolic Acid in Plasma/SerumOrder no.: 46110/HRMycophenolic Acid in Plasma/Serum - HPLC
HPLC Column Mycophenolic Acid in Plasma/SerumOrder no.: 46110/HRMycophenolic Acid in Plasma/Serum - HPLC