One Step CDT in Serum with Fast Elution - HPLC
With ready-to-use pre-mixed tubes
Only one pipetting step
Detects genetic variants
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746
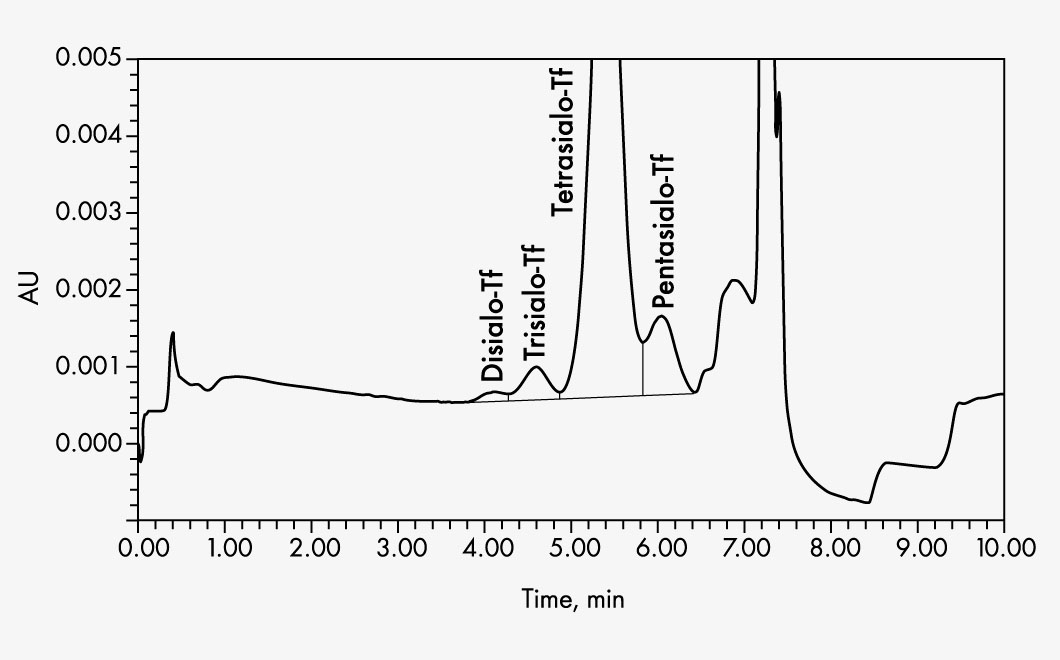

Asialotransferrin
Disialotransferrin
Pentasialotransferrin
Tetrasialotransferrin
Trisialotransferrin
Clinical relevance
CDT (carbohydrate-deficient transferrin) is a lab marker showing the highest specificity for chronic alcohol abuse. CDT values increase significantly with the daily intake of more than 60 g ethanol (the equivalent of 0.75 l wine) per day over a period of two weeks. The values return to normal within two weeks in case of alcohol abstention. This makes CDT an excellent parameter for continuous monitoring in withdrawal programmes or for assessments in forensic or occupational medicine.
Unlike other biochemical parameters like γ-GT or MCV, liver diseases will not cause any false-positive results.
Product advantages
- Very easy sample preparation with pre-mixed tubes
- Only one pipetting step required
- Detection of genetic variants
This Chromsystems assay allows for the fast and reliable measurement of CDT in serum using HPLC and UV detection. Sample preparation with premixed tubes is performed in a single pipetting step, making it fast and convenient.
The CDT is displayed as an area percentage of total transferrin, so it remains unaffected by fluctuations in the concentration of transferrin in serum. Optimised chromatographic separation of transferrin isoforms also allows for the reliable detection of known genetic variants.
A manual method with automated sample preparation is also available as an alternative for greater sample throughput.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 500 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | approx. 0.5 % Disialotransferrin |
| Intraassay | CV = 2.4 % (with 3.3 % Disialo) |
| Interassay | CV = 3.2 % (with 3.3 % Disialo) |
| Specimen | Serum |
| Sample Preparation |
|
| Run Time | 10 min |
| Injection Volume | 150-200 µl |
| Flow Rate | Detection: 1.5 ml/min, Rinsing: 2.5 ml/min |
| Column Temperature | Ambient (~25 °C) |
| Wavelengths | 460 nm |
| Additional Info | For the Chromsystems HPLC analysis of CDT in serum with fast elution chromatography a ternary HPLC gradient system with UV detection is necessary. |
| Parameters | Asialotransferrin, Disialotransferrin, Pentasialotransferrin, Tetrasialotransferrin, Trisialotransferrin |
-
 Mobile Phase AOrder no.: 54931
Mobile Phase AOrder no.: 54931Automated CDT in Serum using 96 Well Filter Plates and One Step CDT in Serum - HPLC
-
 Mobile Phase BOrder no.: 54932
Mobile Phase BOrder no.: 54932Automated CDT in Serum using 96 Well Filter Plates and One Step CDT in Serum - HPLC
-
 Mobile Phase COrder no.: 54933
Mobile Phase COrder no.: 54933Automated CDT in Serum using 96 Well Filter Plates and One Step CDT in Serum - HPLC


Asialotransferrin
Disialotransferrin
Pentasialotransferrin
Tetrasialotransferrin
Trisialotransferrin
Clinical relevance
CDT (carbohydrate-deficient transferrin) is a lab marker showing the highest specificity for chronic alcohol abuse. CDT values increase significantly with the daily intake of more than 60 g ethanol (the equivalent of 0.75 l wine) per day over a period of two weeks. The values return to normal within two weeks in case of alcohol abstention. This makes CDT an excellent parameter for continuous monitoring in withdrawal programmes or for assessments in forensic or occupational medicine.
Unlike other biochemical parameters like γ-GT or MCV, liver diseases will not cause any false-positive results.
Product advantages
- Very easy sample preparation with pre-mixed tubes
- Only one pipetting step required
- Detection of genetic variants
This Chromsystems assay allows for the fast and reliable measurement of CDT in serum using HPLC and UV detection. Sample preparation with premixed tubes is performed in a single pipetting step, making it fast and convenient.
The CDT is displayed as an area percentage of total transferrin, so it remains unaffected by fluctuations in the concentration of transferrin in serum. Optimised chromatographic separation of transferrin isoforms also allows for the reliable detection of known genetic variants.
A manual method with automated sample preparation is also available as an alternative for greater sample throughput.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 500 |
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Lower Limit of Quantitation | approx. 0.5 % Disialotransferrin |
| Intraassay | CV = 2.4 % (with 3.3 % Disialo) |
| Interassay | CV = 3.2 % (with 3.3 % Disialo) |
| Specimen | Serum |
| Sample Preparation |
|
| Run Time | 10 min |
| Injection Volume | 150-200 µl |
| Flow Rate | Detection: 1.5 ml/min, Rinsing: 2.5 ml/min |
| Column Temperature | Ambient (~25 °C) |
| Wavelengths | 460 nm |
| Additional Info | For the Chromsystems HPLC analysis of CDT in serum with fast elution chromatography a ternary HPLC gradient system with UV detection is necessary. |
| Parameters | Asialotransferrin, Disialotransferrin, Pentasialotransferrin, Tetrasialotransferrin, Trisialotransferrin |
-
 Mobile Phase AOrder no.: 54931
Mobile Phase AOrder no.: 54931Automated CDT in Serum using 96 Well Filter Plates and One Step CDT in Serum - HPLC
-
 Mobile Phase BOrder no.: 54932
Mobile Phase BOrder no.: 54932Automated CDT in Serum using 96 Well Filter Plates and One Step CDT in Serum - HPLC
-
 Mobile Phase COrder no.: 54933
Mobile Phase COrder no.: 54933Automated CDT in Serum using 96 Well Filter Plates and One Step CDT in Serum - HPLC






