MassTox® Immunosuppressants in Whole Blood – ONEMinute Test - LC-MS/MS
For sample preparation with 96 Deep Well Extraction Plates
Short run time
Isotopically labelled internal standard for each analyte
6PLUS1® Multilevel Calibrator Set and MassCheck® controls
Additional trap column for removing interfering substances
Assay validated according to IVDR (=> Declaration of Conformity)
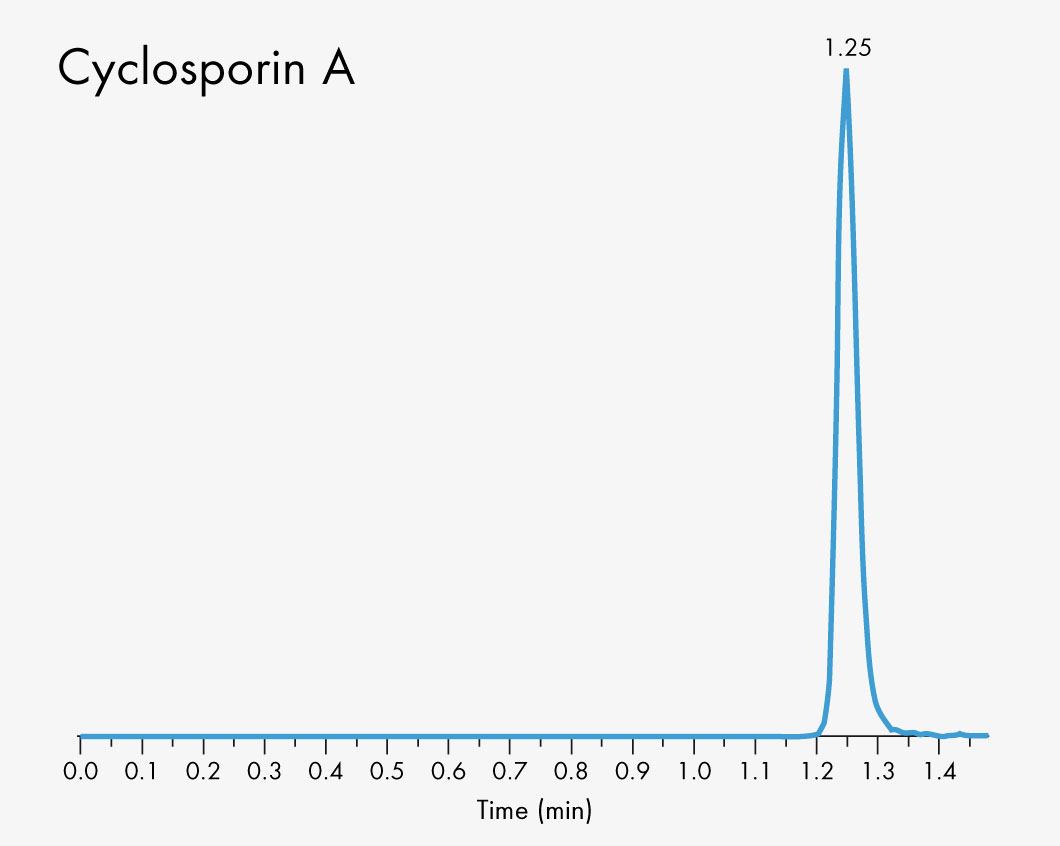

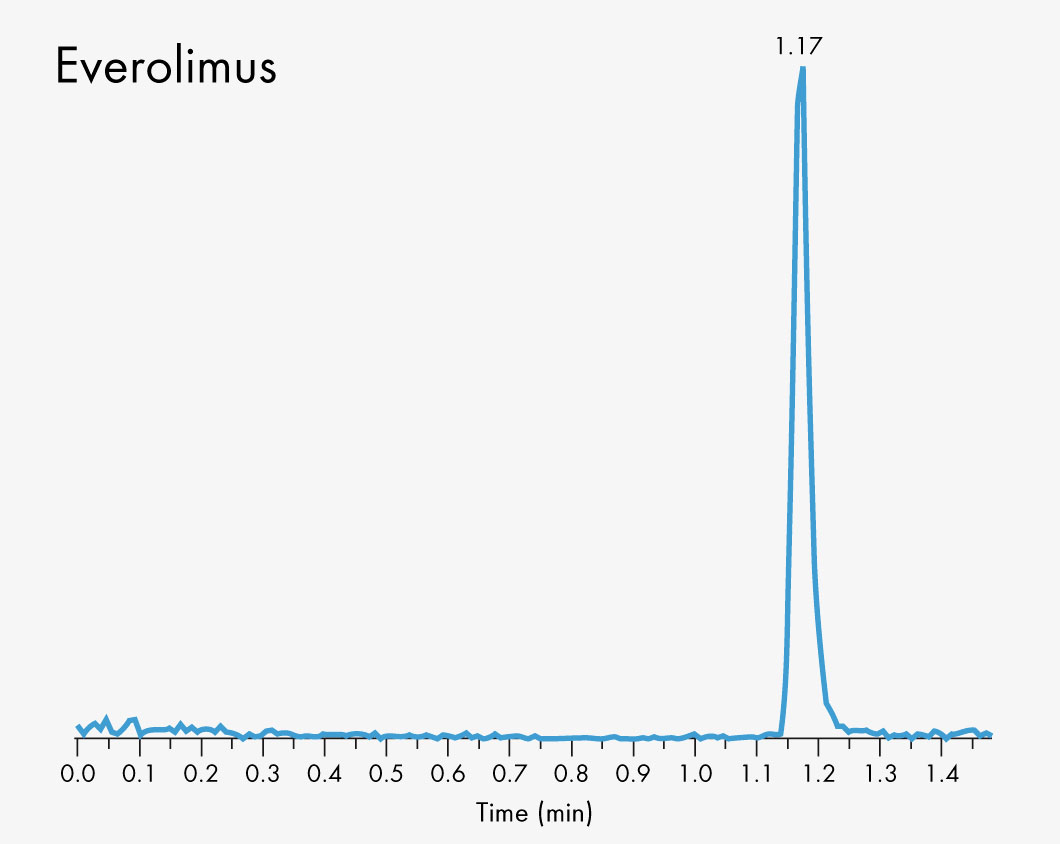

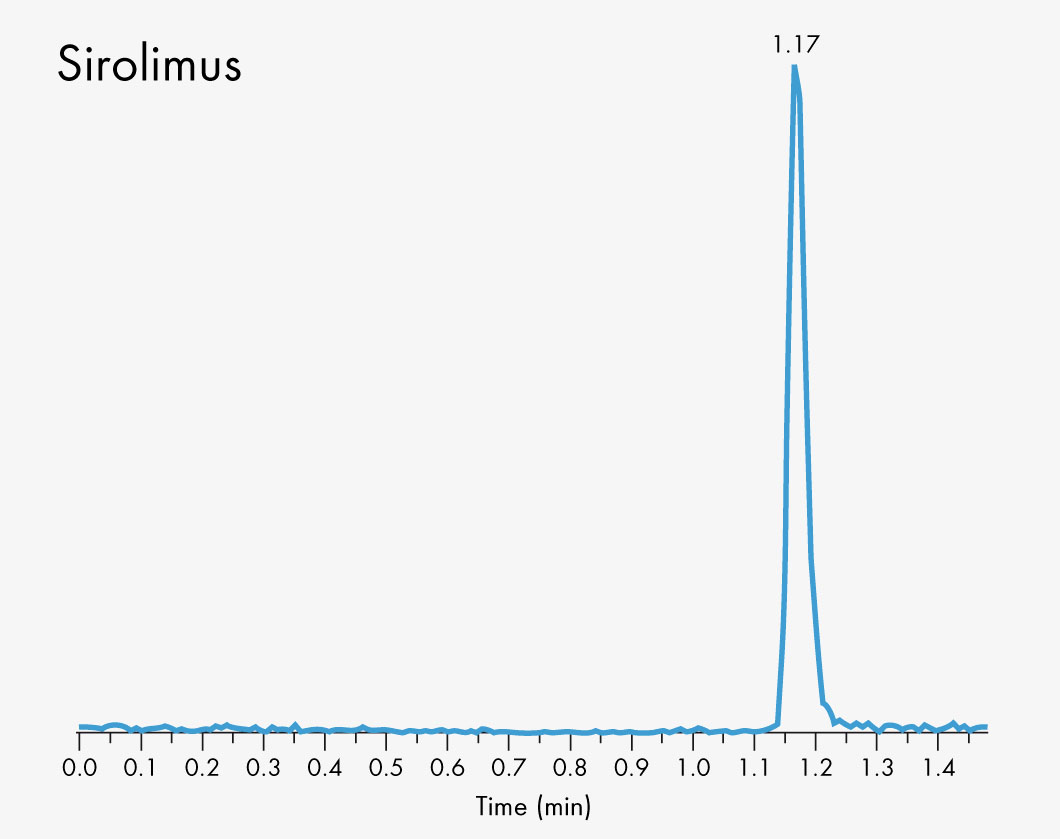

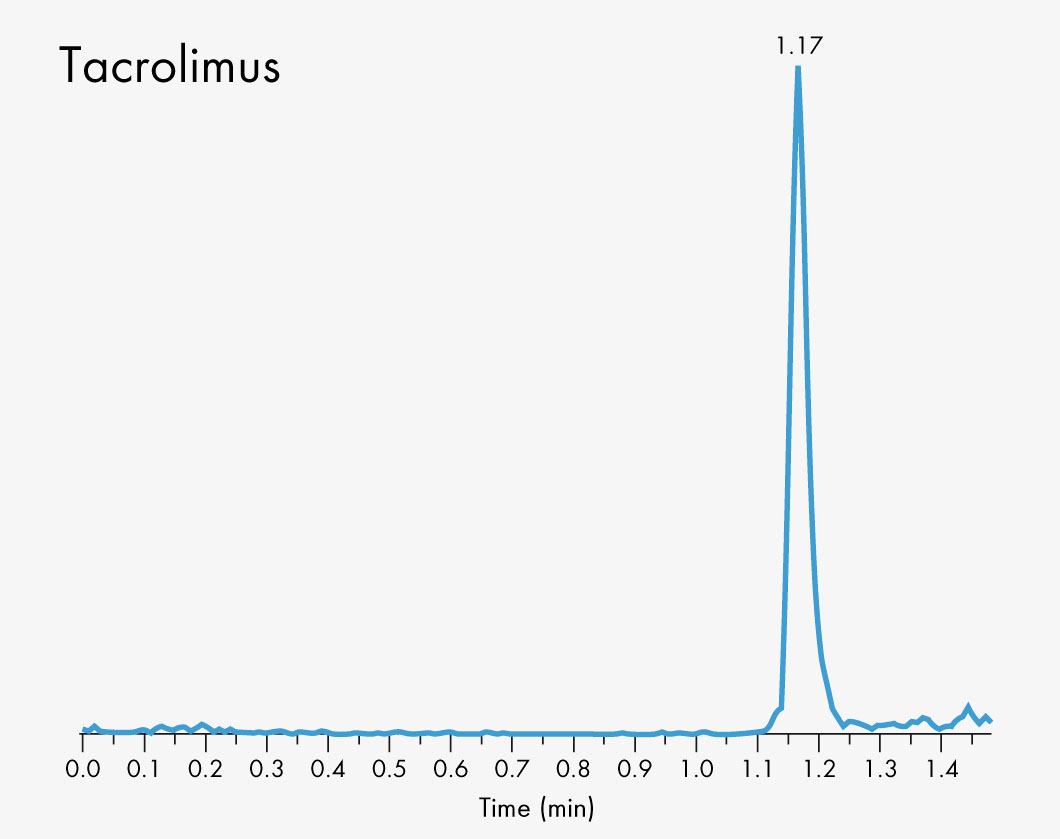

Cyclosporin A
Everolimus
Sirolimus
Tacrolimus
MassChrom® Immunosuppressants in Whole Blood – ONEMinute Test by LC-MS/MS
For sample preparation with 96 Deep Well Extraction Plates
Kit for 1200 tests
Clinical Relevance
This assay allows the quantitative determination of cyclosporin A, everolimus, sirolimus (rapamycin) and tacrolimus (FK-506) in human EDTA whole blood samples via liquid chromatography-mass spectrometry (LC-MS/MS).
It is intended as a therapeutic drug monitoring test for patients treated with one or several immunosuppressants named above.
Product advantages
• Short run time
• Isotopically labelled internal standard for each analyte
• 6PLUS1® Multilevel Calibrator Set and MassCheck® controls
• Additional trap column for removing interfering substances
Assay characteristics
The manual preparation of the sample is limited to a simple and effective protein precipitation process. The analytes are enriched, and interfering substances removed via a trap column. Chromatographic separation is then performed by an analytical column connected to a 2-position 6-port, or 2-position 10-port valve. The use of isotopically-labelled internal standards ensures the precision and robustness of the method and compensates for matrix effects (such as ion suppression).
Detailed performance evaluation data for this assay can be found in the appendix of the instruction manual.
| Method of Analysis | LC-MS/MS |
|---|---|
| Number of Tests | 1200 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data for this assay can be found in the appendix of the instruction manual. |
| Lower and Upper Limit of Quantitation | Cyclosporin A: 6.0 – 2000 µg/l Different systems might show different performance data. |
| Specimen | EDTA Whole Blood |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
| Run Time | 1 – 2 min |
| Injection Volume | 1 – 50 µl |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Parameters | Cyclosporin A, Everolimus, Sirolimus, Tacrolimus |
93900/1200/DWP MassChrom® Immunosuppressants in Whole Blood – ONEMinute Test
For sample preparation with 96 Deep Well Extraction Plates
Kit for 1200 tests
| Order No. | Kit components (Quantities in kit) |
| 93911 | Mobile Phase A, 900 ml (5x) |
| 93922 | Mobile Phase B, 450 ml (3x) |
| 93909 | Rinsing Solution, 1000 ml (2x) |
| 93003 | Precipitation Reagent, 100 ml (3x) |
| 93936 | Internal Standard Set (3x) consisting of: Internal Standard Mix (lyoph.), 4 x 2.5 ml and Reconstitution Buffer, 10 ml |
| 93005 | Extraction Buffer, 40 ml (3x) |
| 93956 | 96 Deep Well Extraction Plates, 5 pcs. (3x) |
| 93058 | 96 Well Collection Plates , 5 pcs. (3x) |
| 93059 | Pierceable Adhesive Seals for 96 Well Plates, 5 pcs. (3x) |
| 28039/XL | 6PLUS1® Multilevel Whole Blood Calibrator Set Immunosuppressants, 7 x 2 ml (1x) |
| 93100 | Analytical Column, equilibrated, with test chromatogram, 1 pc. (1x) |
| 93122 | Trap Column, equilibrated, with test chromatogram, 1 pc. (1x) |
| Order No. | Required components (not included in kit) |
| 0082 | MassCheck® Immunosuppressants Whole Blood Control Level I, 5 x 2 ml |
| 0083 | MassCheck® Immunosuppressants Whole Blood Control Level II, 5 x 2 ml |
| 0084 | MassCheck® Immunosuppressants Whole Blood Control Level III, 5 x 2 ml |
| 0085 | MassCheck® Immunosuppressants Whole Blood Control Level IV, 5 x 2 ml |
| 0089 | MassCheck® Immunosuppressants Whole Blood Blank Control, 5 x 2 ml |
| 93925 | Tuning Mix Immunosuppressants, Analytes and Internal Standards, 1 ml |
| 93060 | Pierceable Heat Seals for 96 Well Plates, 6 pcs. (can be used as an alternative to 93059 - Pierceable Adhesive Seals for 96 Well Plates) |
| Order No. | Lab Equipment |
| 42740 | Chromsystems Heat Sealer, 1 pc. |
| 15010 | PEEK Prefilter Housing, 1 pc. |
| 15011 | PEEK-encased Prefilter, 2 µm, 5 pcs. |
| 15070 | Stainless Steel Prefilter Housing, 1 pc. |
| 15071 | Stainless Steel Prefilter, 0,5 µm, 5 pcs. |
-
 96 Deep Well Extraction PlatesOrder no.: 93956
96 Deep Well Extraction PlatesOrder no.: 93956MassTox® Immunosuppressants ONEMinute Test - LC-MS/MS
-
 Pierceable Adhesive Seals for 96 Well PlatesOrder no.: 93059
Pierceable Adhesive Seals for 96 Well PlatesOrder no.: 93059MassTox® Immunosuppressants ONEMinute Test - LC-MS/MS
-
 6PLUS1® Multilevel Whole Blood Calibrator Set ImmunosuppressantsOrder no.: 28039/XLMassTox® Immunosuppressants in Whole Blood – LC-MS/MS
6PLUS1® Multilevel Whole Blood Calibrator Set ImmunosuppressantsOrder no.: 28039/XLMassTox® Immunosuppressants in Whole Blood – LC-MS/MS -
 Analytical Column Immunosuppressants in Whole Blood - LC-MS/MSOrder no.: 93100
Analytical Column Immunosuppressants in Whole Blood - LC-MS/MSOrder no.: 93100MassTox® Immunosuppressants in Whole Blood - LC-MS/MS
-
 Tuning Mix Immunosuppressants ONEMinute TestOrder no.: 93925MassTox® Immunosuppressants ONEMinute Test - LC-MS/MS
Tuning Mix Immunosuppressants ONEMinute TestOrder no.: 93925MassTox® Immunosuppressants ONEMinute Test - LC-MS/MS -
 Pierceable Heat Seals for 96 Well PlatesOrder no.: 93060MassTox® Immunosuppressants ONEMinute Test for sample preparation with 96 Deep Well Extraction Plates
Pierceable Heat Seals for 96 Well PlatesOrder no.: 93060MassTox® Immunosuppressants ONEMinute Test for sample preparation with 96 Deep Well Extraction Plates
-
 6PLUS1® Multilevel Whole Blood Calibrator Set ImmunosuppressantsOrder no.: 28039/XLMassTox® Immunosuppressants in Whole Blood – LC-MS/MS
6PLUS1® Multilevel Whole Blood Calibrator Set ImmunosuppressantsOrder no.: 28039/XLMassTox® Immunosuppressants in Whole Blood – LC-MS/MS
-
 Analytical Column Immunosuppressants in Whole Blood - LC-MS/MSOrder no.: 93100
Analytical Column Immunosuppressants in Whole Blood - LC-MS/MSOrder no.: 93100MassTox® Immunosuppressants in Whole Blood - LC-MS/MS
-
 Heat SealerOrder no.: 42740
Heat SealerOrder no.: 42740Easy sealing of 96 well plates
Adjustable time and temperature
Ergonomic lever
Compatible with a wide range of microplates
Fast start-up
Small footprint








Cyclosporin A
Everolimus
Sirolimus
Tacrolimus
MassChrom® Immunosuppressants in Whole Blood – ONEMinute Test by LC-MS/MS
For sample preparation with 96 Deep Well Extraction Plates
Kit for 1200 tests
Clinical Relevance
This assay allows the quantitative determination of cyclosporin A, everolimus, sirolimus (rapamycin) and tacrolimus (FK-506) in human EDTA whole blood samples via liquid chromatography-mass spectrometry (LC-MS/MS).
It is intended as a therapeutic drug monitoring test for patients treated with one or several immunosuppressants named above.
Product advantages
• Short run time
• Isotopically labelled internal standard for each analyte
• 6PLUS1® Multilevel Calibrator Set and MassCheck® controls
• Additional trap column for removing interfering substances
Assay characteristics
The manual preparation of the sample is limited to a simple and effective protein precipitation process. The analytes are enriched, and interfering substances removed via a trap column. Chromatographic separation is then performed by an analytical column connected to a 2-position 6-port, or 2-position 10-port valve. The use of isotopically-labelled internal standards ensures the precision and robustness of the method and compensates for matrix effects (such as ion suppression).
Detailed performance evaluation data for this assay can be found in the appendix of the instruction manual.
| Method of Analysis | LC-MS/MS |
|---|---|
| Number of Tests | 1200 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data for this assay can be found in the appendix of the instruction manual. |
| Lower and Upper Limit of Quantitation | Cyclosporin A: 6.0 – 2000 µg/l Different systems might show different performance data. |
| Specimen | EDTA Whole Blood |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
| Run Time | 1 – 2 min |
| Injection Volume | 1 – 50 µl |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Parameters | Cyclosporin A, Everolimus, Sirolimus, Tacrolimus |
93900/1200/DWP MassChrom® Immunosuppressants in Whole Blood – ONEMinute Test
For sample preparation with 96 Deep Well Extraction Plates
Kit for 1200 tests
| Order No. | Kit components (Quantities in kit) |
| 93911 | Mobile Phase A, 900 ml (5x) |
| 93922 | Mobile Phase B, 450 ml (3x) |
| 93909 | Rinsing Solution, 1000 ml (2x) |
| 93003 | Precipitation Reagent, 100 ml (3x) |
| 93936 | Internal Standard Set (3x) consisting of: Internal Standard Mix (lyoph.), 4 x 2.5 ml and Reconstitution Buffer, 10 ml |
| 93005 | Extraction Buffer, 40 ml (3x) |
| 93956 | 96 Deep Well Extraction Plates, 5 pcs. (3x) |
| 93058 | 96 Well Collection Plates , 5 pcs. (3x) |
| 93059 | Pierceable Adhesive Seals for 96 Well Plates, 5 pcs. (3x) |
| 28039/XL | 6PLUS1® Multilevel Whole Blood Calibrator Set Immunosuppressants, 7 x 2 ml (1x) |
| 93100 | Analytical Column, equilibrated, with test chromatogram, 1 pc. (1x) |
| 93122 | Trap Column, equilibrated, with test chromatogram, 1 pc. (1x) |
| Order No. | Required components (not included in kit) |
| 0082 | MassCheck® Immunosuppressants Whole Blood Control Level I, 5 x 2 ml |
| 0083 | MassCheck® Immunosuppressants Whole Blood Control Level II, 5 x 2 ml |
| 0084 | MassCheck® Immunosuppressants Whole Blood Control Level III, 5 x 2 ml |
| 0085 | MassCheck® Immunosuppressants Whole Blood Control Level IV, 5 x 2 ml |
| 0089 | MassCheck® Immunosuppressants Whole Blood Blank Control, 5 x 2 ml |
| 93925 | Tuning Mix Immunosuppressants, Analytes and Internal Standards, 1 ml |
| 93060 | Pierceable Heat Seals for 96 Well Plates, 6 pcs. (can be used as an alternative to 93059 - Pierceable Adhesive Seals for 96 Well Plates) |
| Order No. | Lab Equipment |
| 42740 | Chromsystems Heat Sealer, 1 pc. |
| 15010 | PEEK Prefilter Housing, 1 pc. |
| 15011 | PEEK-encased Prefilter, 2 µm, 5 pcs. |
| 15070 | Stainless Steel Prefilter Housing, 1 pc. |
| 15071 | Stainless Steel Prefilter, 0,5 µm, 5 pcs. |
-
 96 Deep Well Extraction PlatesOrder no.: 93956
96 Deep Well Extraction PlatesOrder no.: 93956MassTox® Immunosuppressants ONEMinute Test - LC-MS/MS
-
 Pierceable Adhesive Seals for 96 Well PlatesOrder no.: 93059
Pierceable Adhesive Seals for 96 Well PlatesOrder no.: 93059MassTox® Immunosuppressants ONEMinute Test - LC-MS/MS
-
 6PLUS1® Multilevel Whole Blood Calibrator Set ImmunosuppressantsOrder no.: 28039/XLMassTox® Immunosuppressants in Whole Blood – LC-MS/MS
6PLUS1® Multilevel Whole Blood Calibrator Set ImmunosuppressantsOrder no.: 28039/XLMassTox® Immunosuppressants in Whole Blood – LC-MS/MS -
 Analytical Column Immunosuppressants in Whole Blood - LC-MS/MSOrder no.: 93100
Analytical Column Immunosuppressants in Whole Blood - LC-MS/MSOrder no.: 93100MassTox® Immunosuppressants in Whole Blood - LC-MS/MS
-
 Tuning Mix Immunosuppressants ONEMinute TestOrder no.: 93925MassTox® Immunosuppressants ONEMinute Test - LC-MS/MS
Tuning Mix Immunosuppressants ONEMinute TestOrder no.: 93925MassTox® Immunosuppressants ONEMinute Test - LC-MS/MS -
 Pierceable Heat Seals for 96 Well PlatesOrder no.: 93060MassTox® Immunosuppressants ONEMinute Test for sample preparation with 96 Deep Well Extraction Plates
Pierceable Heat Seals for 96 Well PlatesOrder no.: 93060MassTox® Immunosuppressants ONEMinute Test for sample preparation with 96 Deep Well Extraction Plates
-
 6PLUS1® Multilevel Whole Blood Calibrator Set ImmunosuppressantsOrder no.: 28039/XLMassTox® Immunosuppressants in Whole Blood – LC-MS/MS
6PLUS1® Multilevel Whole Blood Calibrator Set ImmunosuppressantsOrder no.: 28039/XLMassTox® Immunosuppressants in Whole Blood – LC-MS/MS
-
 Analytical Column Immunosuppressants in Whole Blood - LC-MS/MSOrder no.: 93100
Analytical Column Immunosuppressants in Whole Blood - LC-MS/MSOrder no.: 93100MassTox® Immunosuppressants in Whole Blood - LC-MS/MS
-
 Heat SealerOrder no.: 42740
Heat SealerOrder no.: 42740Easy sealing of 96 well plates
Adjustable time and temperature
Ergonomic lever
Compatible with a wide range of microplates
Fast start-up
Small footprint