Automating LC-MS/MS in Therapeutic Drug Monitoring
LC-MS-based testing offers accuracy, specificity, and a high speed of analysis, which is why it is being adopted across many clinical laboratories. This is particularly true in therapeutic drug monitoring where many drugs need to be tested, and where a narrow therapeutic range often requires a high precision of analysis.
Although most systems are currently non-IVD, automation also appears on the horizon since a while. The interest is high, given that automated sample preparation for LC-MS/MS can significantly increase the throughput in the laboratory, while personnel can focus on other, more valuable tasks. The need for high reproducibility and traceability, as well as the elimination of manual errors is equally important in clinical laboratories for moving into automation.


Hamilton joined up with Chromsystems to develop a platform that is specifically suited for clinical diagnostics and which also takes into account regulatory requirements in clinical laboratories. The result of this collaboration is MassSTAR that combines the best of both worlds - a reliable automation solution from Hamilton together with validated Chromsystems assays into a CE-IVD conforming automation workflow that is ideally suited for therapeutic drug monitoring in the clinical routine.
![]() To learn more about automated sample preparation with the MassSTAR, please click here.
To learn more about automated sample preparation with the MassSTAR, please click here.
More than 100 drugs with one chromatographic setup
The automated assay is based on MassTox® TDM Series A from Chromsystems, a modular system that consists of three components: the specific parameters sets, the chromatographic column, and the BASIC Kit A (see Table). The column itself allows the efficient monitoring of nearly 200 parameters without column switching or change of the mobile phases. In combination with an almost identical sample preparation workflow throughout all parameter sets, this helps to minimise the workload in the laboratory. Furthermore, LC-MS/MS allows multi-analyte measurements with many parameters determined with a single run. Nearly all parameters are safeguarded by internal standards, ensuring a high accuracy of data. Multilevel Calibrator Sets and MassCheck® plasma controls are also part of the solution, which ensure high accuracy of data and traceability of results. So far, more than 100 parameters can be automated in a complete CE-IVD workflow on the MassSTAR with more to follow (for the complete test menu click here). The sample preparation time varies depending on the dilution factor, 96 samples need approximately 90 min total run time, of which about 80 min is walk-away time.
The three components of MassTox® TDM Series A
MasterColumn® A: Analytical column for all parameters
Parameter Sets: 200 parameters divided into 13 parameter sets; each containing individual calibrators, controls and internal standards. 7 parameter sets for more than 100 parameters are automated on MassSTAR so far.
Basic Kit A: All common components required for sample preparation and mobile phases.
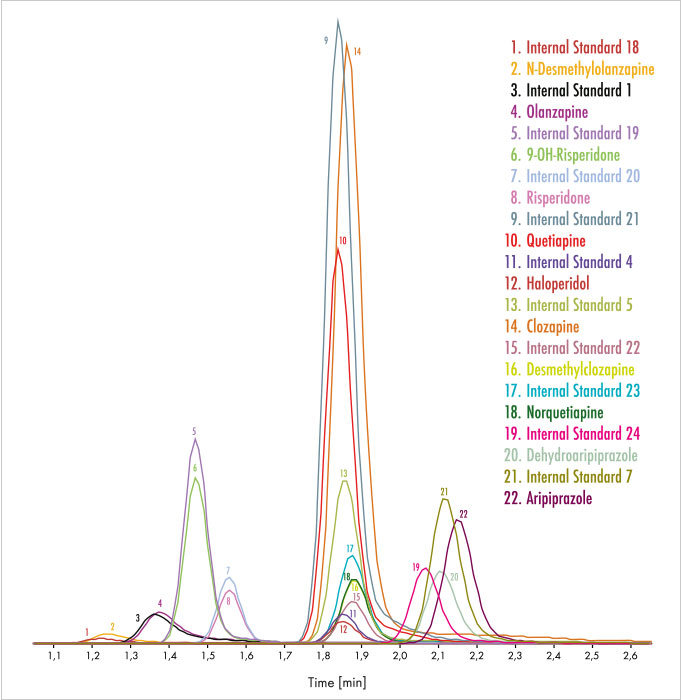
Figure 1: Chromatogram of the Parameter Set Neuroleptics 1/Extended with 11 parameters with one injection.

Automation in a Regulatory Environment
At this point, most systems that automate the sample preparation for LC-MS/MS analysis do not consider the regulatory requirements that clinical laboratories need to conform with. In Europe, the new In Vitro Diagnostics Regulation (IVDR) also came on top with a tremendous impact in the ways clinical laboratories have to work. As a future-proof solution for clinical laboratories, an automation system together with its assays certainly does need to conform with regulatory requirements, which is why the workflows were developed to be CE-IVD compliant. A certification according to IVDR is also in preparation. This requires an enormous effort from R&D and regulatory alike, however, laboratories can rely on a solution that remains up to date with all regulatory needs that laboratories need to fulfil.
Efficiency beyond the sample preparation procedure
Which other requirements does an automation system need to offer for clinical laboratories? When introducing and running new automation processes in a clinical laboratory, one key component is simplicity of use. For MassTox® TDM Series A and any future expansions, Chromsystems has developed a graphical user interface, or short GUI, that guides the user through the loading process along with an inventory check. This includes barcode matching, a check if the plates are correctly positioned, and a verification of appropriate liquid levels for the reagents. This means that all reagents and consumables are available in sufficient quantities before the run is started ensuring that no further user intervention is required, thereby providing additional walk-away time. Behind this are sophisticated, autonomous error handling algorithms. Descriptive screens monitor the current state of the sample preparation process and show the steps the system will perform next. The GUI also prevents invalid user inputs as a protection against unauthorised manipulation. Additionally, a tailor-made reagent carrier for MassTox® TDM Series enables the loading of assay reagents in their original containers. This means that the reagents do not have to be transferred, saving time, preventing contaminations and a mixing up of reagents, as well as lowering evaporation of the liquid. Processed samples and reagents can be unloaded during the run for optimised time management in the laboratory. For example, as soon as the patient samples have been pipetted into the plate, they can be removed from the system, even if the workflow is still running.
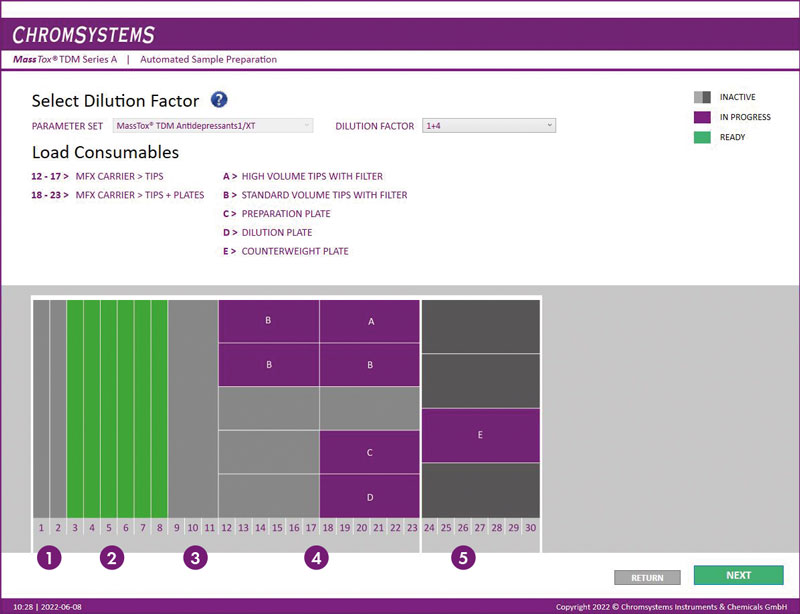

Figure 2: Software interface. For an intuitive user experience, the layout is identical to the actual deck layout of MassSTAR.
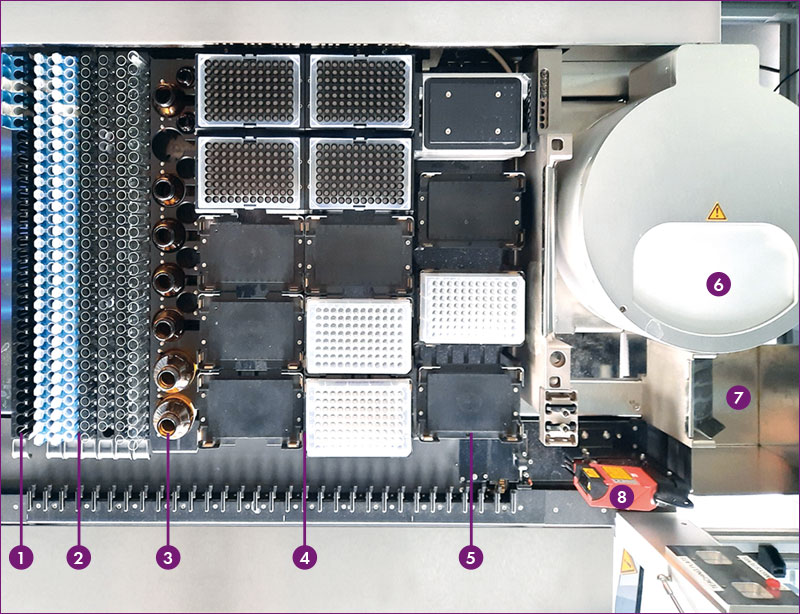

Figure 3: Deck layout for MassTox® TDM Series A
1 Carrier for calibrators and controls, 2 Carrier for sample tubes, 3 Chromsystems reagent carrier, 4 Carrier for tips and 96 (deep-) well plates, 5 Carrier for (heater-) shaker and counterweight plate, 6 Centrifuge, 7 Waste, 8 Barcode reader
Consistency of data
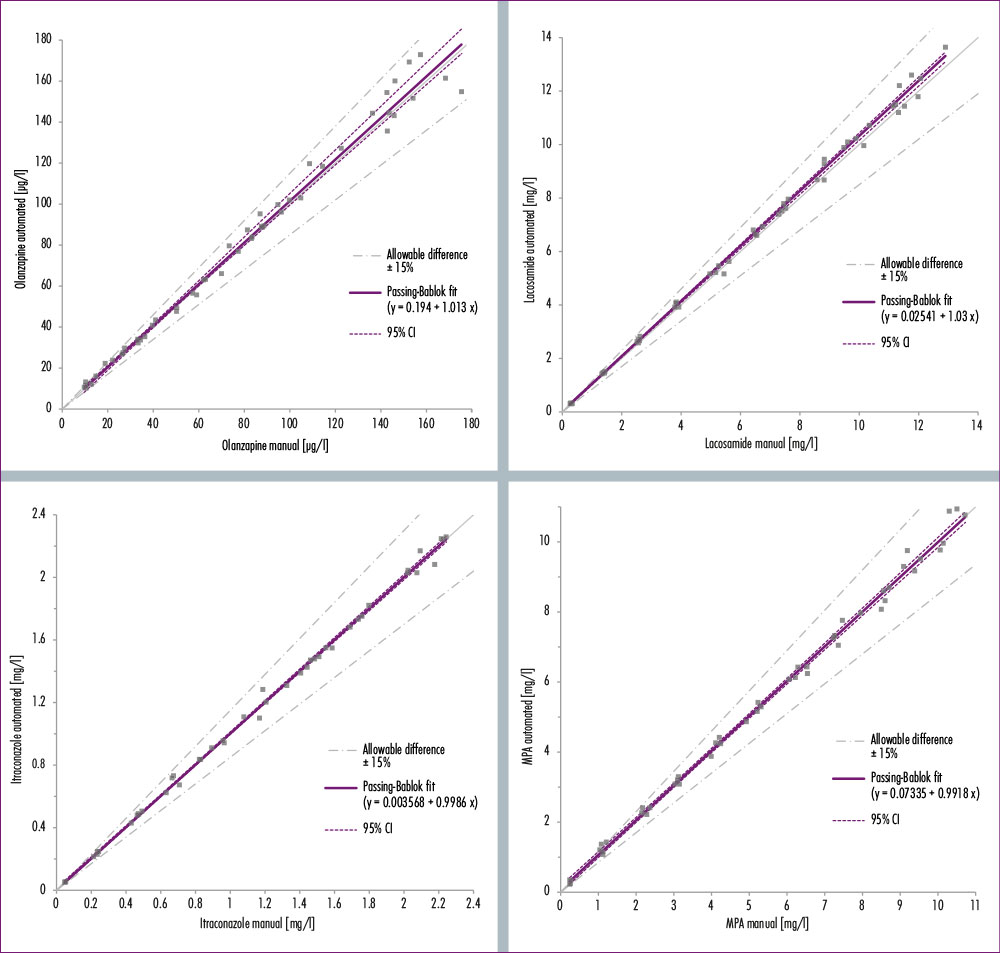
One concern laboratories may have when switching from manual to automated sample preparation processes is consistency of data. Inconsistencies can cause problems in the interpretation of the results. This is why method comparisons between manual and automated sample preparation for all analytes have been performed for MassSTAR that clearly demonstrate a high correlation of data. In essence: same results, lower risk of pipetting errors, increased throughput and reduced costs.
Figure 4: Pasing-Bablock regression comparing manual and automated sample preparation for the four analytes olanzapine, lacosamide, itraconazole and mycophenpolic acid (MPA). The graphs demonstrate a high correlation of data.


Safer than Manual
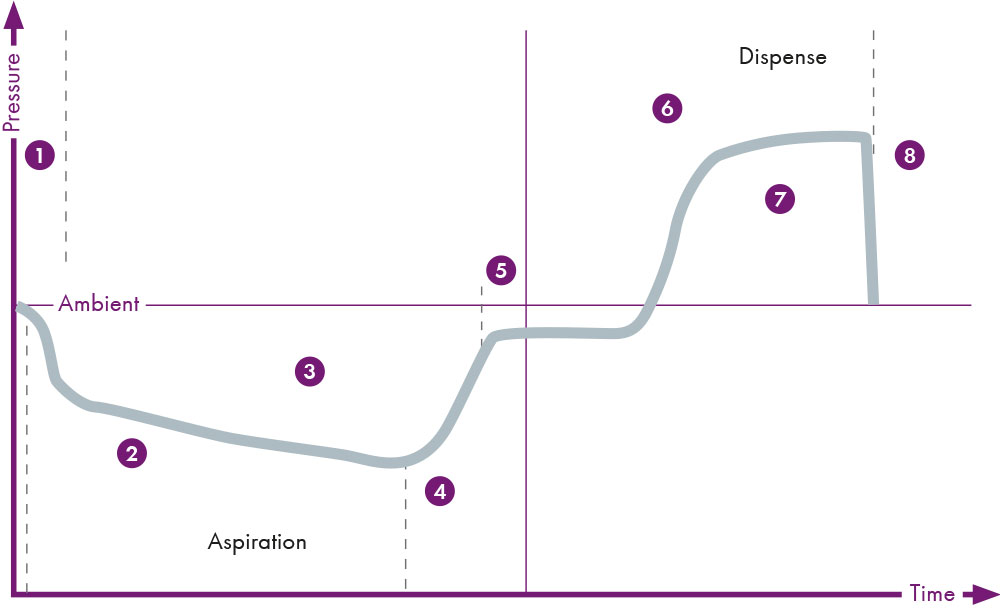
Automation systems can reduce the risk of pipetting errors compared to the manual sample preparation process, but an automation system such as MassSTAR can do much more. It can perform sensory monitoring of all pipetting steps – including clot and foam detection. This is based on one of the key safety features of MassSTAR: the total aspiration and dispense monitoring (TADM™). The pressure in each pipetting channel is monitored during each aspiration and dispensing step in real-time. TADM™ verifies the sample transfer with a traceable digital audit trail and identifies errors such as blood clots or an incorrect volume that has been transferred. In case an error during aspiration or dispensing is detected, the system handles it by internal error handling. Conspicuous samples are then excluded.
Figure 5: TADM verifies aspiration and dispensing, ensuring that clots and other issues are identified and reported (Grey line indicates a typical pressure profile).
1 Measurement not working, 2 Blocked tip, blood clot, 3 Short sample, 4 Incorrect value, 5 Short/no sample, 6 Dispense speed incorrect/blocked tip, 7 System leak, 8 Incorrect volume
Summary
LC-MS/MS is considered the gold standard for therapeutic drug monitoring with high accuracy, specificity and a high speed of analysis. Therefore, the interest in automation in clinical laboratories is high. MassSTAR in conjunction with Chromsystems assays offers an automated CE-IVD workflow for more than 100 drugs, immunosuppressants, and vitamin D. MassSTAR is a turnkey solution that has been developed with clinical laboratories in mind, offering user comfort, regulatory conformity and flexibility demanded by clinical laboratories. Instrumentation and assays are comprehensively supported by the Chromsystems scientific support team, which has comprehensive experience in clinical LC-MS/MS and automation. This ensures a smooth transition when moving from manual to automated sample preparation for LC-MS/MS analysis.
Last Update 18th of November 2022
The Parameter Menu automated on MassSTAR
Do you have feedback to this article?
