Therapeutic Drug Monitoring of Immunosuppressants with MassTox® Series A
Introduction
Therapeutic drug monitoring of immunosuppressants is indispensable for therapy following organ transplantation. Cyclosporin A, everolimus, sirolimus and tacrolimus are mainly prescribed for avoiding organ rejection. Laboratories working with MassTox® TDM Series A can perform therapeutic drug monitoring of more than 200 drugs with one column and an identical sample preparation. So far, it excluded the analysis of immunosuppressants. Here, we describe that MassTox® Series A can also be used for the analysis of cyclosporin A, everolimus, sirolimus and tacrolimus
Material and Methods
Sample Preparation
The sample preparation was performed in line with the MassTox® Immunosuppressants protocol (Chromsystems). In brief: 50 µl of sample (EDTA-whole blood), reconstituted 6PLUS1® calibrator (order no 28039/XL) or MassCheck® control (order no 0081-0085, 0089) was pipetted into a 1.5 ml reaction vial. 25 µl of the reconstituted Internal Standard Mix (order no. 93946/RUO) and 100 µl Extraction Buffer (order no 93005/RUO) were added and mixed briefly. 250 µl of Precipitation Reagent (order no. 93003/RUO) was added, vortexed for 1 min and incubated for 2 min at ambient temperature, then centrifuged for 5 min at 15000 g. The supernatant was diluted with Dilution Buffer 1 (92007/RUO; ratio 1:1).
LC-MS/MS
10 – 50 µl were injected at a temperature for the autosampler of 8°C – 15°C. Substances were separated on MasterColumn® A (order. no 92110) with a column temperature of 70°C. Flow rate, position of selection valve and binary gradient can be found in table 1. Detection was performed with electrospray ionisation in positive ion mode with a Sciex 4500 mass spectrometer. Used multiple reaction monitoring (MRM) transitions are found in table 2.
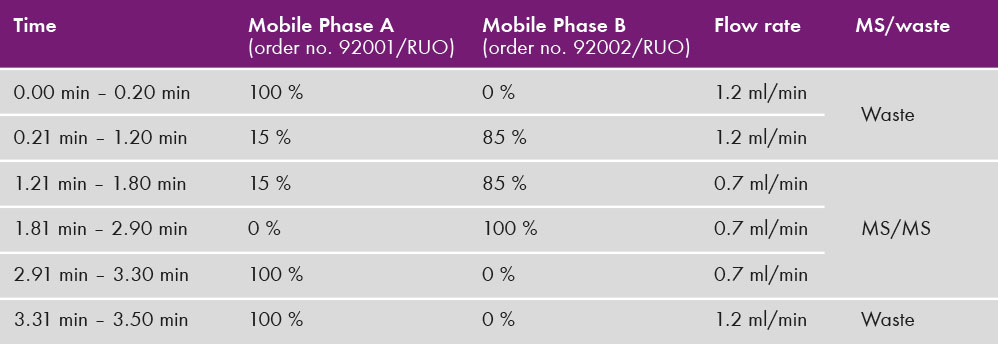

Table 1: Binary gradient profile
Quantification
A full calibration of the analysis system for each series of measurements has been performed with 6PLUS1® Multilevel Calibrator Set (order no. 28039/XL). Calibration curves were constructed by calculating the analyte to internal standard (ISTD) peak area ratio on the y axis against calibrator concentrations on the x axis. Then a calibration curve was plotted for all analytes using linear regression and 1/x weighting.
Results
The analysis of the four immunosuppressants was performed with an analysis time of 3.5 min (see chromatogram in fig. 1). Coefficients of variation were below 10% [Inter-assay (CV = 2.1 % - 8.6 %) and intra-assay (CV = 1.9 % - 8.3 %)] for all clinically relevant concentration ranges (see table 3). The lower limit and upper limits of quantification were determined and cover published therapeutic ranges for all four analytes (table 4).
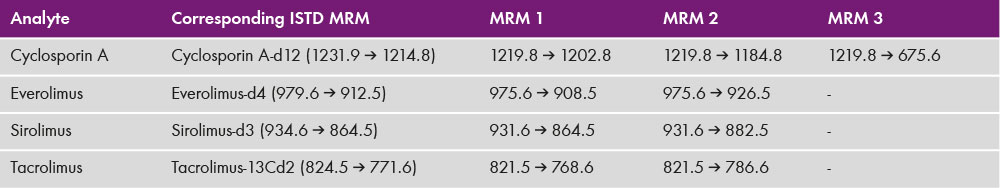

Table 2: MRMs and corresponding ISTD
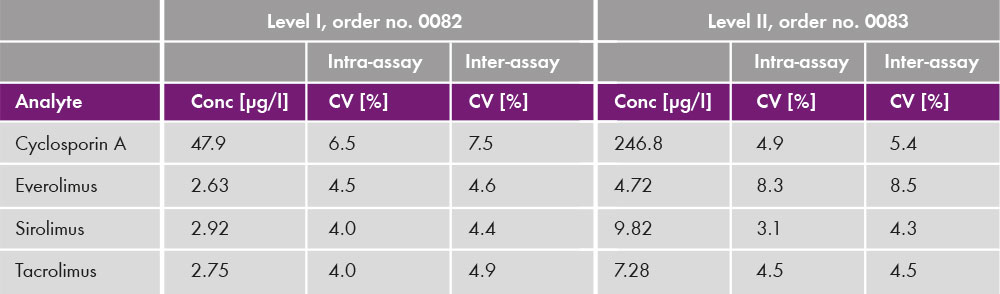

Table 3: Intra- and inter-assay data
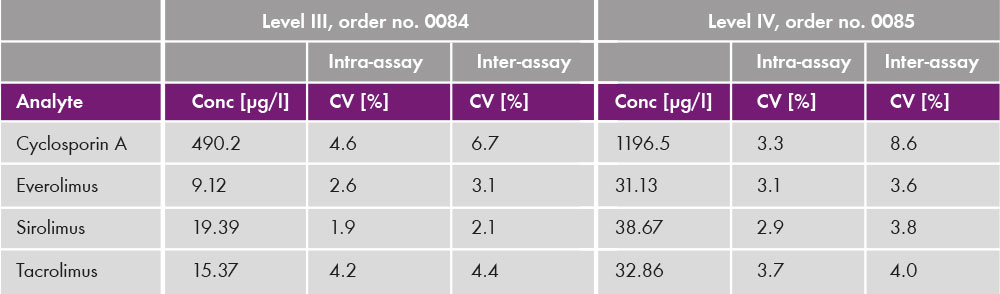

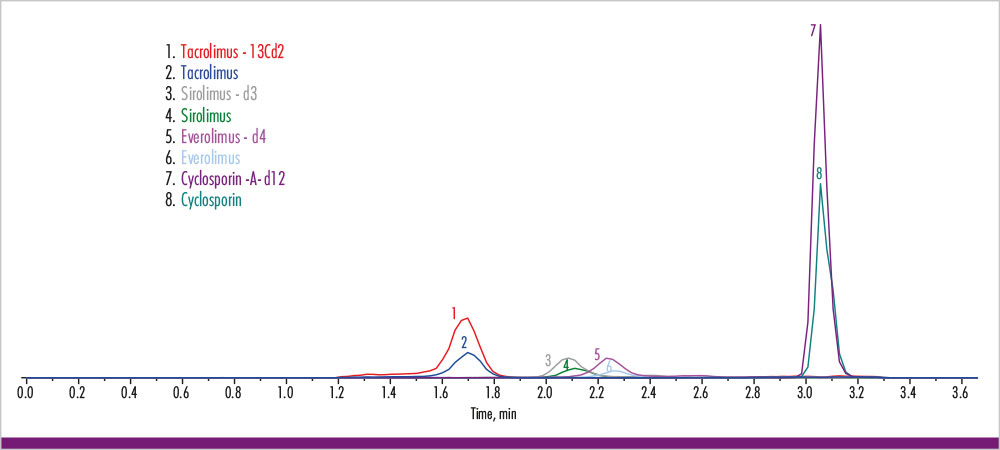

Fig. 1: Chromatogram for the four immunosuppressants and its internal standards.
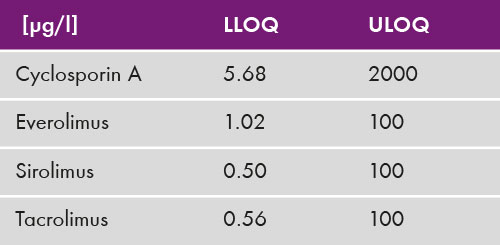

Table 4: Upper and lower limit of quantification
Conclusions
We demonstrate in this application note that the immunosuppressants cyclosporin A, everolimus, sirolimus and tacrolimus in whole blood can be analysed by using a hybrid of the two Chromsystems kits: MassTox® Series A and the MassTox® ONEMINUTE Immunosuppressants assay. The sample preparation is performed in line with the immunosupressants assay and with a dilution using a reagent of Series A. The sample is then applied to the analytical system of MassTox® Series A without modification to the chromatographic setup.
While this approach is not CE-IVD compliant, customers can use this assay on a research-use basis, enabling the analysis of more than 200 drugs plus immunosuppressants in the laboratory while also eliminating the need for changes to the chromatographic setup.
Ordering Information (To order the products or for a quote, please contact us here)
MassTox® Series A
| Order No. | Products |
| 92001/RUO |
Mobile Phase 1 |
| 92002/RUO |
Mobile Phase 2 |
| 92009/RUO |
Rinsing Solution |
| 92110 |
MassTox® TDM Master Column Series A |
| 92007/RUO |
Dilution Buffer 1 |
RUO: For research use only and not for diagnostic purposes
MassTox® Immunosuppressants
| Order No. | Products |
| 29039/XL |
6PLUS1® Multilevel Whole Blood Calibrator Set |
| 0081-0085, 0089 |
MassCheck® Immunosuppressants Whole Blood Controls Level I-IV, Blank |
| 93946/RUO |
Internal Standard Set |
| 93003/RUO |
Precipitation Reagent |
| 93005/RUO |
Extraction Buffer |
| 33006 |
Reaction vials, transparent |
| 93915/RUO |
Tuning Mix |
Last Update 27th October 2021

