UDI labelling of our products
2D code: easier device identification – automatic data scanning
We are currently integrating the new UDI code on our labels as part of the Unique Device Identification (UDI) System. UDIs are required only in the United States so far, but will become mandatory in Europe when the new IVDR comes into effect.
This code allows you to scan key information about our devices electronically.
Examples of UDI codes:


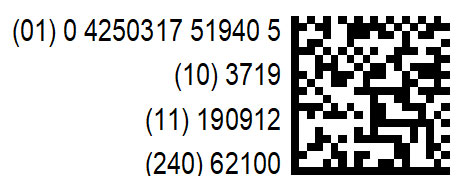

The two-dimensional code replaces our former barcode and contains the following details
| UDI Code | Information | Comment |
| (01) | GTIN (Global Trade Item Number) | Uniquely identifies the item all over the world |
| (10) | Batch number | |
| (17) | Shelf-life | To the exact day in a YYMMDD format |
| (11) | Manufacturing date | To the exact day in a YYMMDD format, If a shelf-life cannot be specified for a particular device, the manufacturing date is given instead. |
| (240) | Chromsystems order number |
The codes (01) (10) (17) and (11) are compulsory for UDIs, the specification of our article number is an additional service for you.
To scan the code, you will need a 2D scanner that is able to scan data matrix formats. A suitably equipped mobile phone also does the job.
Please note:
During the period of transition, your delivery may contain devices with and without UDI coding. The labelling meets all the applicable transition deadlines.
Symbols on our labels - Recognise important information easily
Apart from the UDI code, we introduce further symbols according to EN ISO 15223-1 in addition to the symbols you already know. They are also explained in our instruction manuals.
| Symbol | Meaning | Usage |
| Manufacturer | On all labels (if the size allows) | |
| Manufacturing date | If no expiry date is specified | |
| Sufficient for <n> appliances | On the labels of our assays | |
| Serial number | On the labels of our columns | |
| V1.0 | Version number | Printed close to the 2D Code |
| CE marking of conformity | Conformity assessment by notified body (TÜV Süd Product Service GmbH) |
You already know the following symbols. They have been listed and explained in our instruction manuals for some time.
| Symbol | Meaning | Usage |
| Use by | Products with expiry date | |
| Order number | All products | |
| Batch/Lot code | All IVD products | |
| In-vitro diagnostic medical device | All IVD products | |
| See instructions for use | All IVD products | |
| Upper temperature limit | Store below a certain temperature | |
| Temperature limit | Store within a certain temperature range | |
| Caution | Added for products containing human blood, urine or saliva |
Examples of different labels
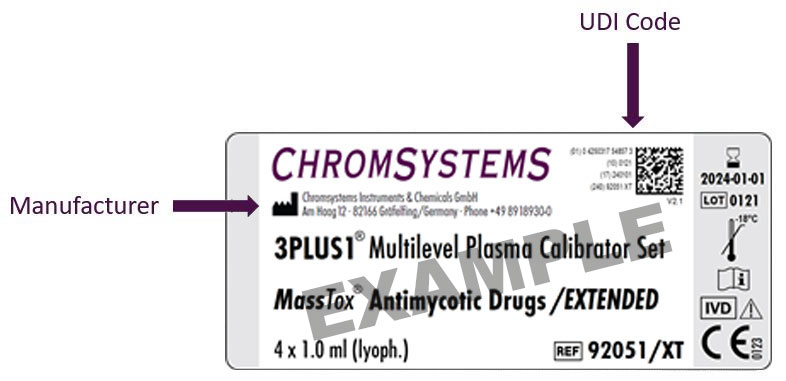

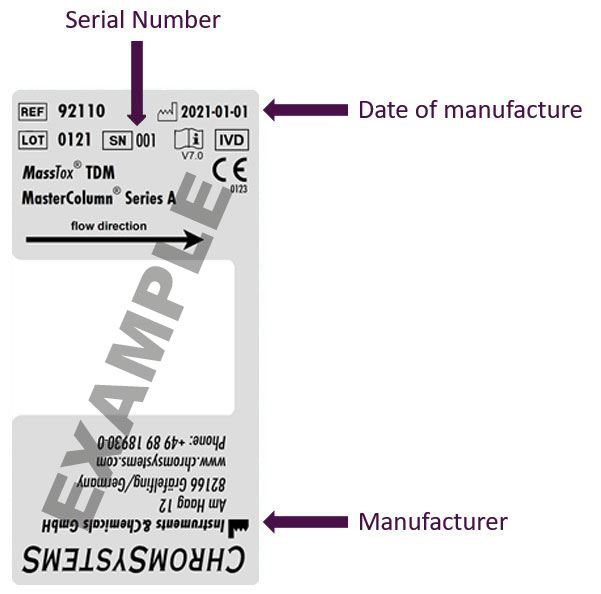

Please note:
During the period of transition, your delivery may contain devices with and without the new symbols. The labelling meets all the applicable transition deadlines.
Latest Update 16th of September 2022
