5-HIAA in Urine - HPLC
Easy sample preparation
Exact pH adjustment not required
The alternative to immunoassay methods
Compatible with the Chromsystems VMA/HVA/5-HIAA method
CE-IVD validated product ready for IVDR within timeframes and transition periods specified by the IVDR 2017/746

5-Hydroxyindoleacetic Acid (5-HIAA)
Clinical relevance
A number of diseases are associated with pathological changes in serotonin metabolism. In carcinoid tumour, a malignant hyperplasia of the enterochromaffin cells of the gastrointestinal tract, there is a marked increase in serotonin release into blood plasma. This tissue hormone is metabolised enzymatically in the liver by monoamine oxidase (MAO) and aldehyde dehydrogenase (ADH) to 5-hydroxyindole-3-acetic acid (5-HIAA) and subsequently excreted via the kidney. Endocrine-active carcinoid thus results in an increased concentration of 5-HIAA in the urine. The quantitative determination of 5-HIAA in urine is therefore used for the diagnosis of the carcinoid tumour and for therapy monitoring.
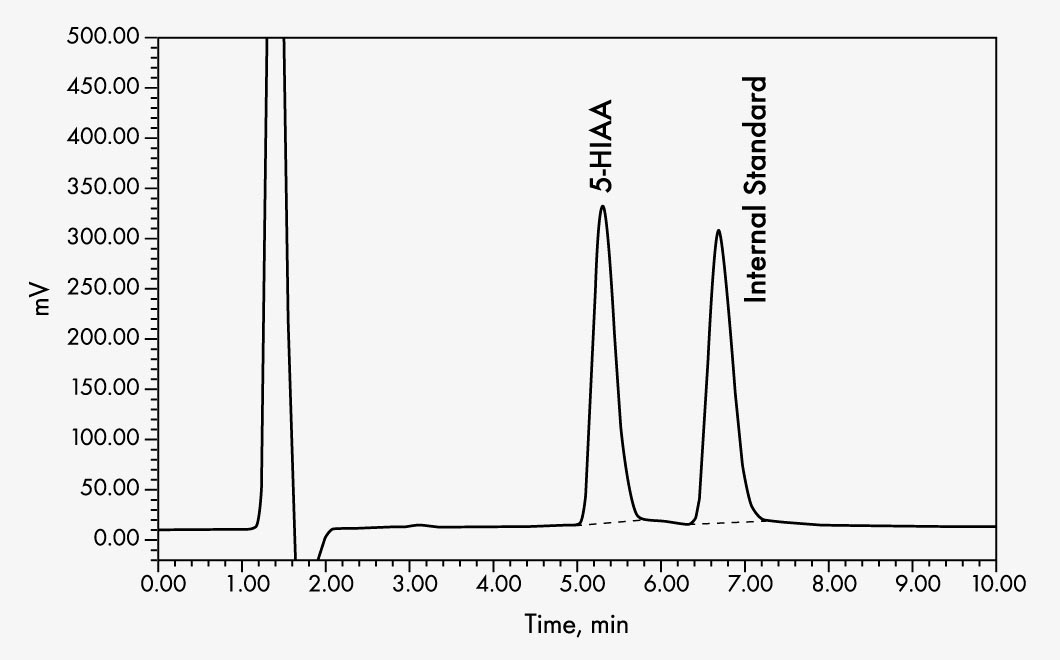
This assay allows the quantitative determination of 5-hydroxyindoleacetic acid (5-HIAA) in human urine samples by HPLC (high performance liquid chromatography) with electrochemical detection.
It is intended as a monitoring test for patients with suspected serotonin-secreting tumours.
Product advantages
• Easy sample preparation
• Exact pH adjustment not required
• The alternative to immunoassay methods
• Compatible with the Chromsystems VMA/HVA/5-HIAA method
Assay characteristics
Urine samples prepared with this assay can also be analysed with column and mobile phase of the VMA/HVA/5-HIAA Chromsystems assay.
Detailed performance evaluation data for this assay can be found in the appendices of the instruction manual.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data for this assay can be found in the appendices of the instruction manual. |
| Lower and Upper Limit of Quantitation | 0.5 mg/l – 25 mg/l |
| Specimen | 24 h-urines collected with 10 ml glacial acetic acid. |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
| Run Time | approx. 9 min |
| Injection Volume | 10-20 µl |
| Flow Rate | 0.8 - 1.3 ml/min |
| Column Temperature | ambient (~25 °C) |
| Potential | +620 to +680 mV |
| Additional Info | For the HPLC analysis 5-HIAA in urine any isocratic HPLC system with electrochemical detector is suitable. |
| Parameters | 5-Hydroxyindoleacetic Acid (5-HIAA) |


5-Hydroxyindoleacetic Acid (5-HIAA)
Clinical relevance
A number of diseases are associated with pathological changes in serotonin metabolism. In carcinoid tumour, a malignant hyperplasia of the enterochromaffin cells of the gastrointestinal tract, there is a marked increase in serotonin release into blood plasma. This tissue hormone is metabolised enzymatically in the liver by monoamine oxidase (MAO) and aldehyde dehydrogenase (ADH) to 5-hydroxyindole-3-acetic acid (5-HIAA) and subsequently excreted via the kidney. Endocrine-active carcinoid thus results in an increased concentration of 5-HIAA in the urine. The quantitative determination of 5-HIAA in urine is therefore used for the diagnosis of the carcinoid tumour and for therapy monitoring.
This assay allows the quantitative determination of 5-hydroxyindoleacetic acid (5-HIAA) in human urine samples by HPLC (high performance liquid chromatography) with electrochemical detection.
It is intended as a monitoring test for patients with suspected serotonin-secreting tumours.
Product advantages
• Easy sample preparation
• Exact pH adjustment not required
• The alternative to immunoassay methods
• Compatible with the Chromsystems VMA/HVA/5-HIAA method
Assay characteristics
Urine samples prepared with this assay can also be analysed with column and mobile phase of the VMA/HVA/5-HIAA Chromsystems assay.
Detailed performance evaluation data for this assay can be found in the appendices of the instruction manual.
| Method of Analysis | HPLC |
|---|---|
| Number of Tests | 100 |
| Please note | The information listed here, including the sample preparation, is not sufficient for using the product. Please read the information provided in the instruction manual, which includes detailed information on limitations associated with the use of the product in line with its intended purpose. Detailed performance evaluation data for this assay can be found in the appendices of the instruction manual. |
| Lower and Upper Limit of Quantitation | 0.5 mg/l – 25 mg/l |
| Specimen | 24 h-urines collected with 10 ml glacial acetic acid. |
| Sample Preparation | The information on the sample preparation presented here is not sufficient for use in the laboratory. For a detailed step by step description, please refer to the instruction manual.
|
| Run Time | approx. 9 min |
| Injection Volume | 10-20 µl |
| Flow Rate | 0.8 - 1.3 ml/min |
| Column Temperature | ambient (~25 °C) |
| Potential | +620 to +680 mV |
| Additional Info | For the HPLC analysis 5-HIAA in urine any isocratic HPLC system with electrochemical detector is suitable. |
| Parameters | 5-Hydroxyindoleacetic Acid (5-HIAA) |