News
Please note: some products are not for diagnostic purposes and for research-use only.


Simple chromatographic method for detecting drugs of abuse in hair
A study presents a simplified LC-MS/MS method for detecting 26 drugs in hair using our MassTox® products. The method offers reduced sample preparation with reliable performance and is well suited for routine laboratory workflows and multiple drugs of abuse screening.


Ensuring accurate vitamin D measurement
Chromsystems participated in a global study to evaluate new vitamin D reference materials and was one of the few laboratories to meet all quality criteria. The results confirm the high quality of our products and the superiority of LC-MS/MS methods.
Article: TDM in ECMO Patients
During extracorporeal membrane oxygenation intensive care patients receive various antibiotics. If these drugs are not measured regularly, then patients are at risk of being underdosed and are unlikely to meet the MIC.
Study: Time to Rethink Amino Acid Analysis (AAA)
Quantitative AAA requires high sensitivity and specificity. A recent study by Carling et al suggests that it might be time to rethink quantitative AAA and compared IEC with LC-MS/MS as an alternative method.
Educational Webinar: Challenging the Status Quo of Amino Acid Analysis in Plasma
Dr. Rachel Carling, Scientific Director for Viapath, reports in this recorded webinar on a study that compares the current gold standard in quantitative amino acid analysis (AAA) - ion exchange chromatography (IEC) - with LC-MS/MS.
Scientific Paper: Vitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2
A new study investigated the potential relationship between vitamin D blood concentrations and SARS-CoV-2 infections. Is the risk of an infection and the disease severity influenced by the vitamin D status? The scientists used the MassChrom® Vitamin D assay on a MassSTAR automation system.
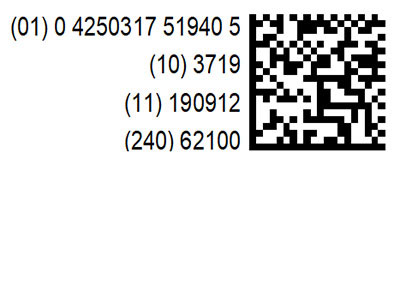
New Labels: Chromsystems is updating its product labels to include UDI
We are already integrating the new UDI code on our labels as part of the Unique Device Identification (UDI) System. UDIs are required only in the United States so far, but will become mandatory in Europe when the new IVDR comes into effect.
Article: Drugs of Abuse Testing with LC-MS/MS
In this article we compare LC-MS/MS with GC-MS currently considered as gold standard. The benefits of LC-MS/MS are demonstrated and it is shown how a 100% hydrolysis of glucuroniated drugs can be achieved.
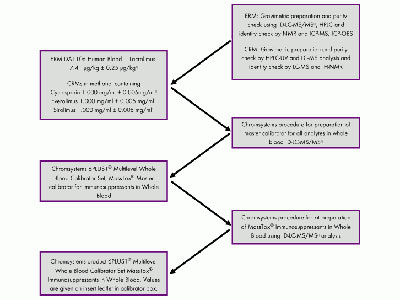
Article: Traceability in Clinical Diagnostics
The ultimate aim of traceability is to ensure that analytical results used for patient care are accurate as well as comparable over time and location. What we do to achieve that? Learn more in this article.
Article: Therapeutic Drug Monitoring in Intensive Care
This article discusses the importance of appropriate drug concentrations in antibiotic therapies to and help prevent over- and underdosing. This approach also reduces the risk of new multidrug resistant strains emerging and helps to provide the most effective drug dose to the patient.