Parameter Set Antiepileptic Drugs - LC-MS/MS
Encompasses 26 analytes
3PLUS1® Multilevel Calibrator Set available
Part of the modular system MassTox® Series A
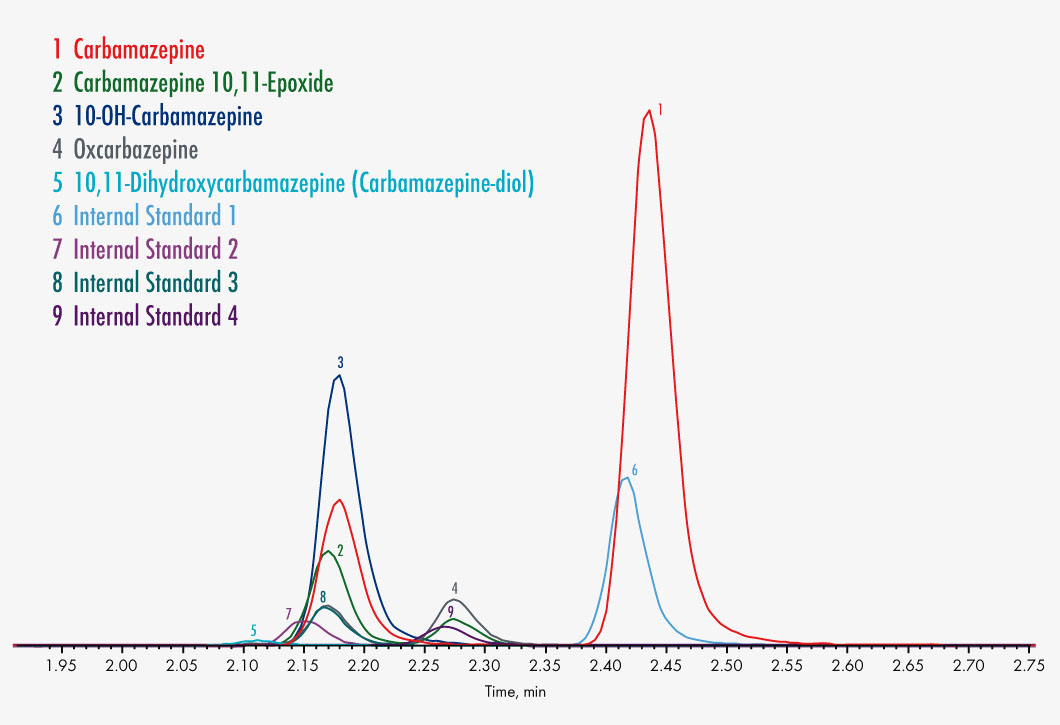

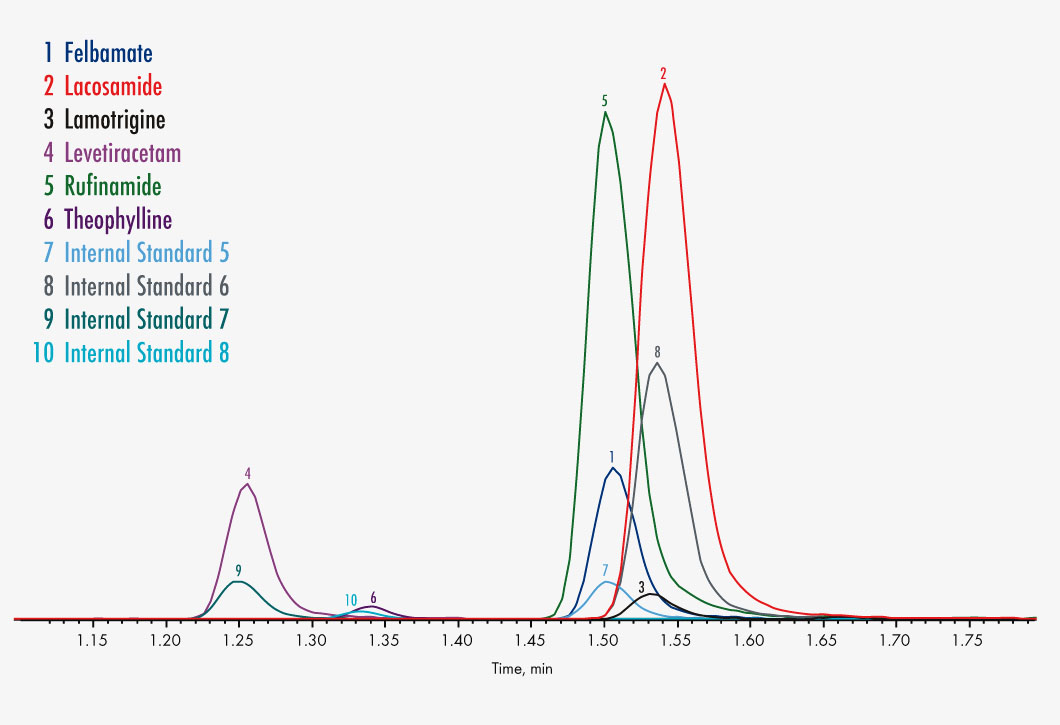

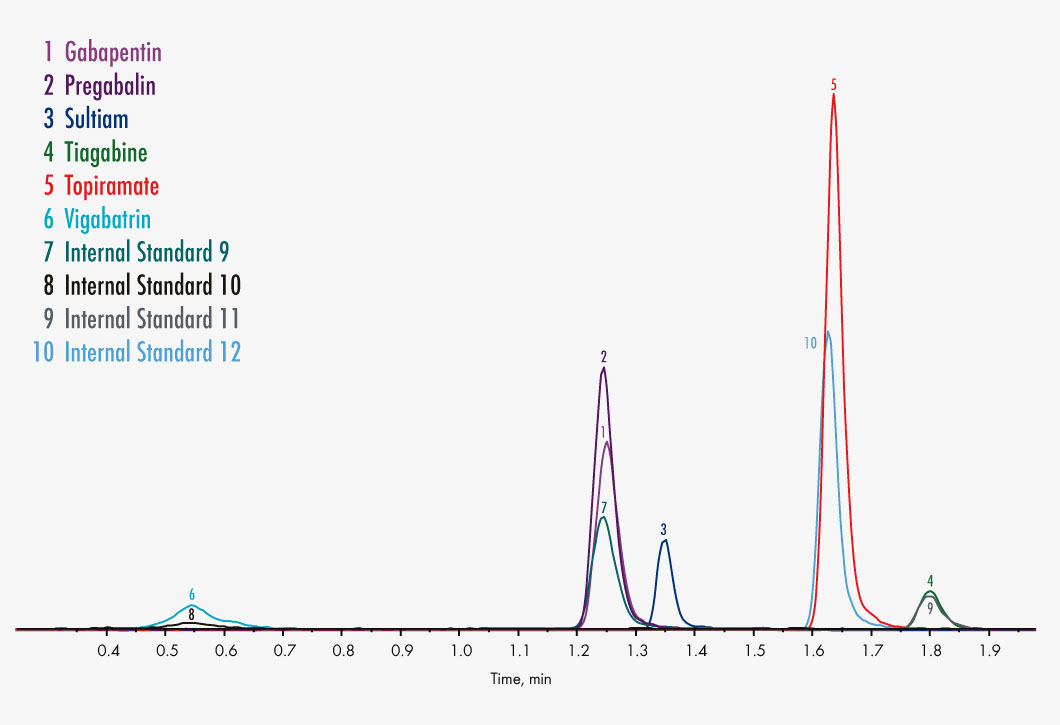

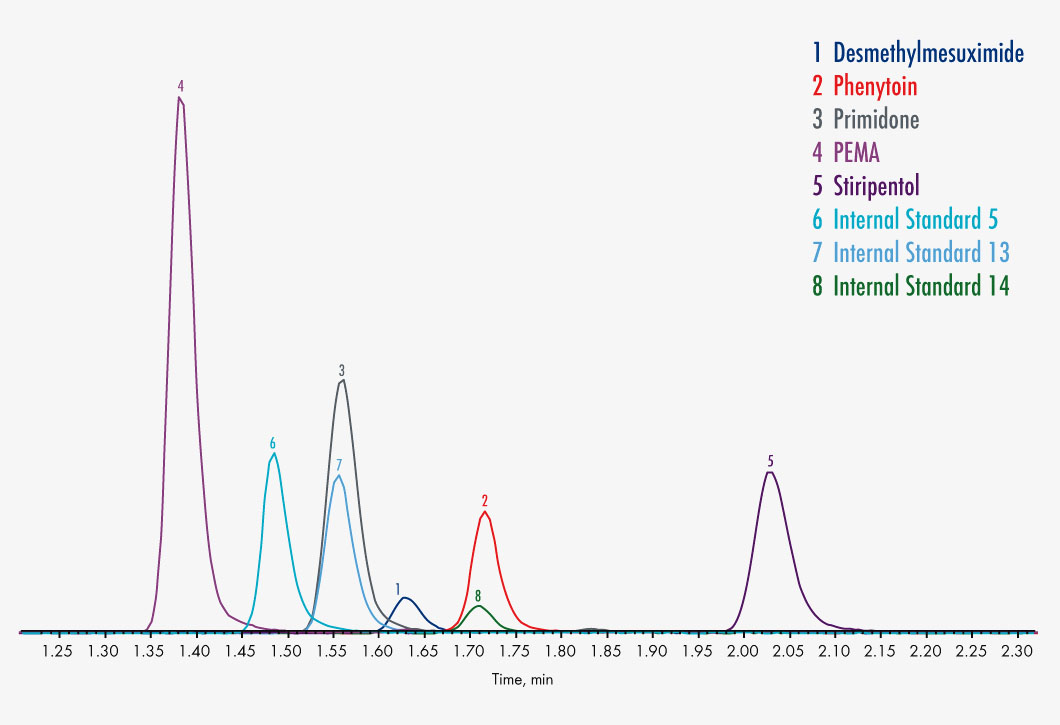


Carbamazepine
Carbamazepine-10,11-epoxide
10,11-Dihydroxycarbamazepine
10-OH-Carbamazepine
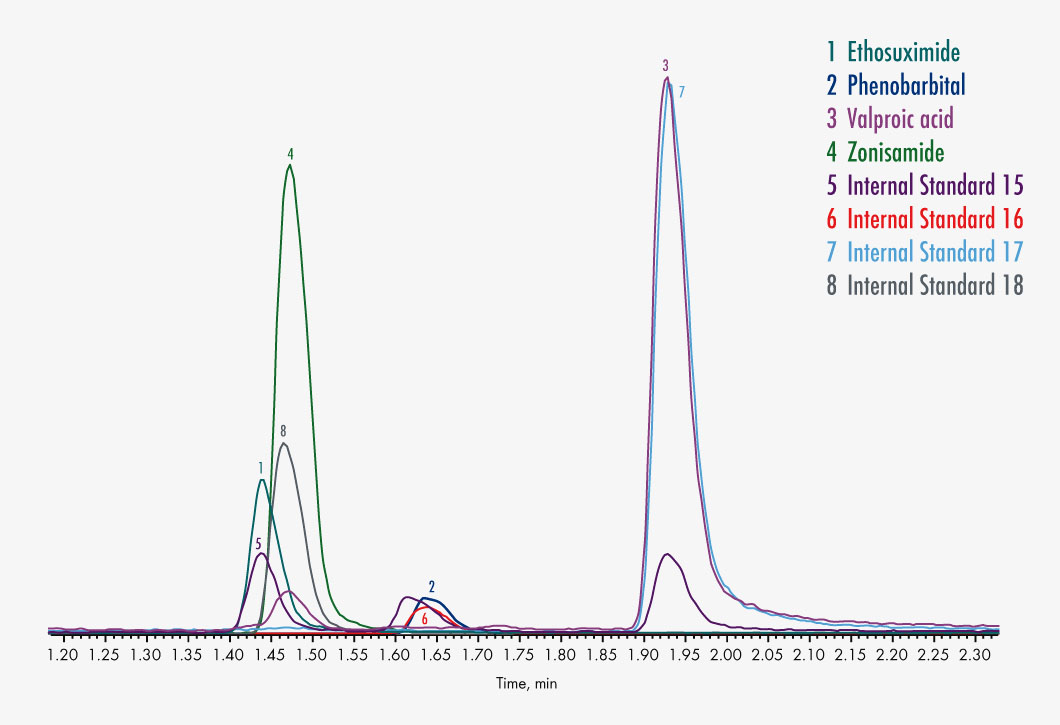
Ethosuximide
Felbamate
Gabapentin
Lacosamide
Lamotrigine
Levetiracetam (Keppra®)
N-Desmethylmesuximide
Oxcarbazepine
Phenobarbital
Phenylethylmalonamide (PEMA)
Phenytoin
Pregabalin
Primidone
Rufinamide
Stiripentol
Sultiame
Theophyllie
Tiagabine
Topiramate
Valproic acid
Vigabatrin
Zonisamid
Clinical relevance
This parameter set allows the quantitative determination of 26 analytes in human serum or plasma samples via liquid chromatography mass spectrometry (LC-MS/MS).
MassTox® Series A
The MassTox® Series A is a modular system that enables the determination of all analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® Basic Kit A
• Specific MassTox® Parameter Set
• Analytical column MassTox® TDM MasterColumn® A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 2.5 – 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Starting point: 0% Mobile Phase 2 Group 1 Group 2 and 3 Group 4 and 5 |
| Ionisation | ESI positive and negative |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | 10-OH-Carbamazepine, Carbamazepine, Ethosuximide, Felbamate, Gabapentin, Lacosamide, Lamotrigine, N-Desmethylmesuximide, Phenobarbital, Phenytoin, Pregabalin, Primidone, Rufinamide, Stiripentol, Sultiame, Theophylline, Tiagabine, Topiramate, Valproic Acid, Vigabatrin, Zonisamide |
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs/EXTENDEDOrder no.: 92025/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs/EXTENDEDOrder no.: 92025/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS -
 MassCheck® Antiepileptic Drugs/EXTENDED Plasma ControlsOrder no.: 0249/XT; 0250/XT; 0251/XT
MassCheck® Antiepileptic Drugs/EXTENDED Plasma ControlsOrder no.: 0249/XT; 0250/XT; 0251/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS
-
 Internal Standard Mix Antiepileptic DrugsOrder no.: 92546/RUOComponent of the Parameter Set Antiepileptic Drugs, available separately
Internal Standard Mix Antiepileptic DrugsOrder no.: 92546/RUOComponent of the Parameter Set Antiepileptic Drugs, available separately
-
 Tuning Mix Antiepileptic Drugs/EXTENDED 1Order no.: 92034/XT/RUOTuning Mix 1 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 1Order no.: 92034/XT/RUOTuning Mix 1 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Tuning Mix Antiepileptic Drugs/EXTENDED 2Order no.: 92035/XT/RUOTuning Mix 2 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 2Order no.: 92035/XT/RUOTuning Mix 2 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Tuning Mix Antiepileptic Drugs/EXTENDED 3Order no.: 92036/XT/RUOTuning Mix 3 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 3Order no.: 92036/XT/RUOTuning Mix 3 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Tuning Mix Antiepileptic Drugs/EXTENDED 4Order no.: 92037/XT/RUOTuning Mix 4 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 4Order no.: 92037/XT/RUOTuning Mix 4 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Tuning Mix Antiepileptic Drugs/EXTENDED 5Order no.: 92038/XT/RUOTuning Mix 5 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 5Order no.: 92038/XT/RUOTuning Mix 5 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200/RUO
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200/RUOPart of the modular system MassTox® Series A
Provides all components required for sample prep and all mobile phases -
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000/RUO
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000/RUOPart of the modular system MassTox® Series A
Provides all components required for sample prep and all mobile phases
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs/EXTENDEDOrder no.: 92025/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs/EXTENDEDOrder no.: 92025/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS
-
 MassCheck® Antiepileptic Drugs/EXTENDED Plasma ControlsOrder no.: 0249/XT; 0250/XT; 0251/XT
MassCheck® Antiepileptic Drugs/EXTENDED Plasma ControlsOrder no.: 0249/XT; 0250/XT; 0251/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS










Carbamazepine
Carbamazepine-10,11-epoxide
10,11-Dihydroxycarbamazepine
10-OH-Carbamazepine
Ethosuximide
Felbamate
Gabapentin
Lacosamide
Lamotrigine
Levetiracetam (Keppra®)
N-Desmethylmesuximide
Oxcarbazepine
Phenobarbital
Phenylethylmalonamide (PEMA)
Phenytoin
Pregabalin
Primidone
Rufinamide
Stiripentol
Sultiame
Theophyllie
Tiagabine
Topiramate
Valproic acid
Vigabatrin
Zonisamid
Clinical relevance
This parameter set allows the quantitative determination of 26 analytes in human serum or plasma samples via liquid chromatography mass spectrometry (LC-MS/MS).
MassTox® Series A
The MassTox® Series A is a modular system that enables the determination of all analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® Basic Kit A
• Specific MassTox® Parameter Set
• Analytical column MassTox® TDM MasterColumn® A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | 2.5 – 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Starting point: 0% Mobile Phase 2 Group 1 Group 2 and 3 Group 4 and 5 |
| Ionisation | ESI positive and negative |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | 10-OH-Carbamazepine, Carbamazepine, Ethosuximide, Felbamate, Gabapentin, Lacosamide, Lamotrigine, N-Desmethylmesuximide, Phenobarbital, Phenytoin, Pregabalin, Primidone, Rufinamide, Stiripentol, Sultiame, Theophylline, Tiagabine, Topiramate, Valproic Acid, Vigabatrin, Zonisamide |
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs/EXTENDEDOrder no.: 92025/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs/EXTENDEDOrder no.: 92025/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS -
 MassCheck® Antiepileptic Drugs/EXTENDED Plasma ControlsOrder no.: 0249/XT; 0250/XT; 0251/XT
MassCheck® Antiepileptic Drugs/EXTENDED Plasma ControlsOrder no.: 0249/XT; 0250/XT; 0251/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS
-
 Internal Standard Mix Antiepileptic DrugsOrder no.: 92546/RUOComponent of the Parameter Set Antiepileptic Drugs, available separately
Internal Standard Mix Antiepileptic DrugsOrder no.: 92546/RUOComponent of the Parameter Set Antiepileptic Drugs, available separately
-
 Tuning Mix Antiepileptic Drugs/EXTENDED 1Order no.: 92034/XT/RUOTuning Mix 1 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 1Order no.: 92034/XT/RUOTuning Mix 1 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Tuning Mix Antiepileptic Drugs/EXTENDED 2Order no.: 92035/XT/RUOTuning Mix 2 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 2Order no.: 92035/XT/RUOTuning Mix 2 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Tuning Mix Antiepileptic Drugs/EXTENDED 3Order no.: 92036/XT/RUOTuning Mix 3 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 3Order no.: 92036/XT/RUOTuning Mix 3 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Tuning Mix Antiepileptic Drugs/EXTENDED 4Order no.: 92037/XT/RUOTuning Mix 4 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 4Order no.: 92037/XT/RUOTuning Mix 4 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Tuning Mix Antiepileptic Drugs/EXTENDED 5Order no.: 92038/XT/RUOTuning Mix 5 for the Parameter Set Antiepileptic Drugs - LC-MS/MS
Tuning Mix Antiepileptic Drugs/EXTENDED 5Order no.: 92038/XT/RUOTuning Mix 5 for the Parameter Set Antiepileptic Drugs - LC-MS/MS -
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200/RUO
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200/RUOPart of the modular system MassTox® Series A
Provides all components required for sample prep and all mobile phases -
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000/RUO
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000/RUOPart of the modular system MassTox® Series A
Provides all components required for sample prep and all mobile phases
-
 3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs/EXTENDEDOrder no.: 92025/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS
3PLUS1® Multilevel Plasma Calibrator Set Antiepileptic Drugs/EXTENDEDOrder no.: 92025/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS
-
 MassCheck® Antiepileptic Drugs/EXTENDED Plasma ControlsOrder no.: 0249/XT; 0250/XT; 0251/XT
MassCheck® Antiepileptic Drugs/EXTENDED Plasma ControlsOrder no.: 0249/XT; 0250/XT; 0251/XTMassTox® Series A Antiepileptic Drugs in Serum/Plasma – LC-MS/MS







