Parameter Set Anti-HIV Drugs - LC-MS/MS
Encompasses 17 analytes
6PLUS1® Multilevel Calibrator Set available
Part of the modular system MassTox® Series A
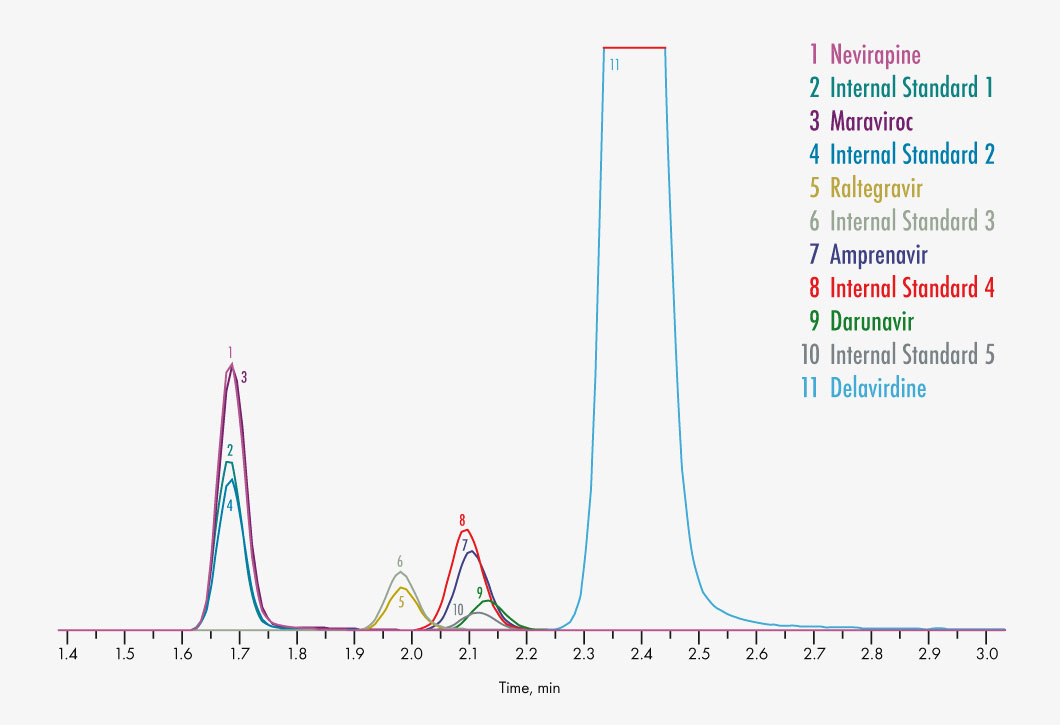

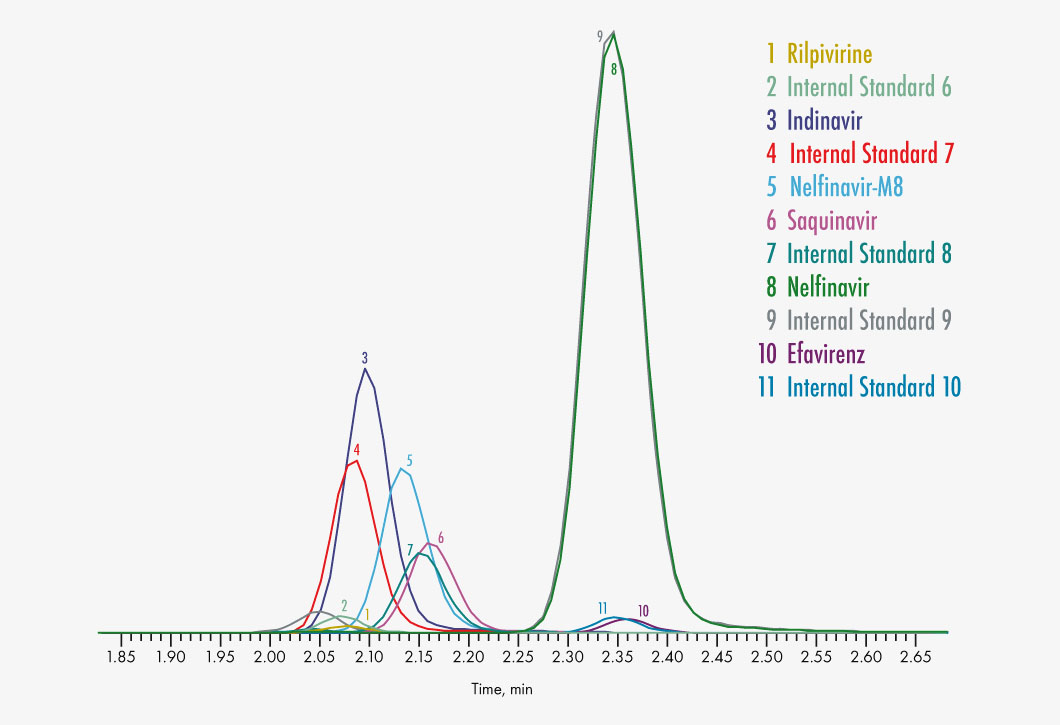

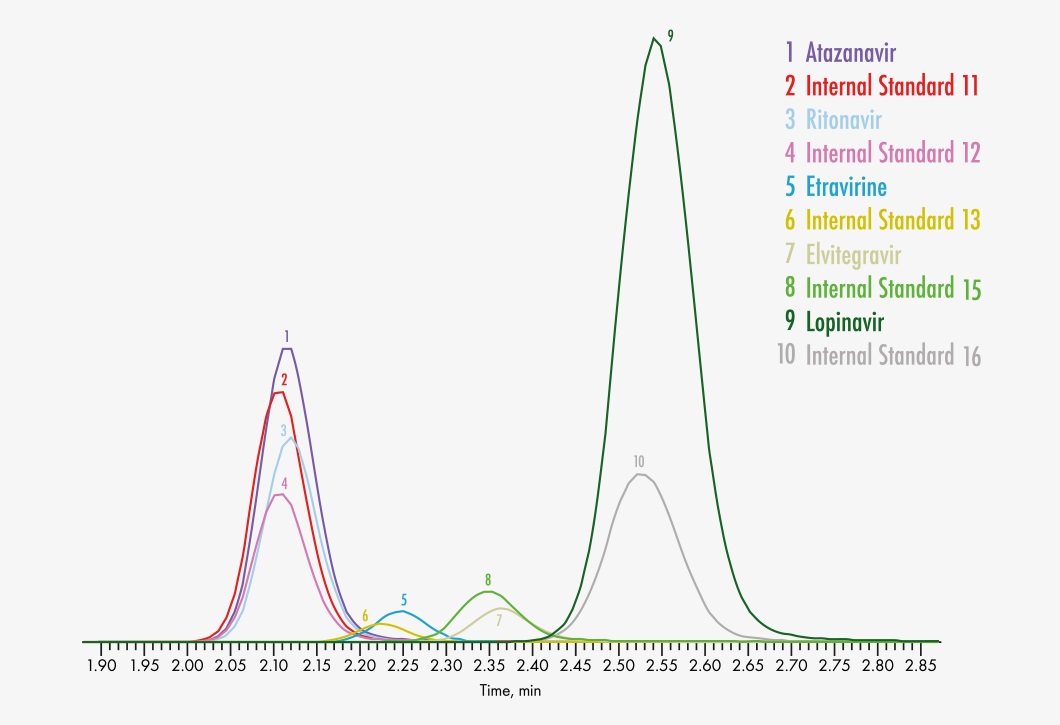

Amprenavir
Atazanavir
Darunavir
Delavirdine
Efavirenz
Elvitegravir
Etravirine
Indinavir
Lopinavir
Maraviroc
Nelfinavir
Nelfinavir-M8
Nevirapine
Raltegravir
Rilpivirine
Ritonavir
Saquinavir
Clinical relevance
This parameter set allows the quantitative determination of amprenavir, atazanavir, darunavir, delavirdine, efavirenz, elvitegravir, etravirine, indinavir, lopinavir, maraviroc, nelfinavir, nelfinavir-M8, nevirapine, raltegravir, rilpivirine, ritonavir and saquinavir in human serum or plasma samples via liquid chromatography mass spectrometry (LC-MS/MS).
MassTox® Series A
The MassTox® Series A is a modular system that enables the determination of all analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® Basic Kit A
• Specific MassTox® Parameter Set
• Analytical column MassTox® TDM MasterColumn® A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | ≤ 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Group 1 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | Amprenavir, Atazanavir, Darunavir, Efavirenz, Elvitegravir, Indinavir, Lopinavir, Maraviroc, Nelfinavir, Nelfinavir-M8, Raltegravir, Ritonavir, Saquinavir |
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS -
 Internal Standard Mix Anti-HIV DrugsOrder no.: 92844/RUOComponent of the Parameter Set Anti-HIV Drugs, available separately
Internal Standard Mix Anti-HIV DrugsOrder no.: 92844/RUOComponent of the Parameter Set Anti-HIV Drugs, available separately
-
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200/RUO
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200/RUOPart of the modular system MassTox® Series A
Provides all components required for sample prep and all mobile phases -
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000/RUO
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000/RUOPart of the modular system MassTox® Series A
Provides all components required for sample prep and all mobile phases
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS






Amprenavir
Atazanavir
Darunavir
Delavirdine
Efavirenz
Elvitegravir
Etravirine
Indinavir
Lopinavir
Maraviroc
Nelfinavir
Nelfinavir-M8
Nevirapine
Raltegravir
Rilpivirine
Ritonavir
Saquinavir
Clinical relevance
This parameter set allows the quantitative determination of amprenavir, atazanavir, darunavir, delavirdine, efavirenz, elvitegravir, etravirine, indinavir, lopinavir, maraviroc, nelfinavir, nelfinavir-M8, nevirapine, raltegravir, rilpivirine, ritonavir and saquinavir in human serum or plasma samples via liquid chromatography mass spectrometry (LC-MS/MS).
MassTox® Series A
The MassTox® Series A is a modular system that enables the determination of all analytes without changing column or mobile phases, thereby minimising the workload in the laboratory.
It consists of 3 parts:
• MassTox® Basic Kit A
• Specific MassTox® Parameter Set
• Analytical column MassTox® TDM MasterColumn® A
| Method of Analysis | LC-MS/MS |
|---|---|
| Please note | The freely available information on this website, in particular on the sample preparation, are not sufficient to work with our products. Please read instructions and warning notices on products and/or instruction manuals. |
| Specimen | Serum/Plasma |
| Sample Preparation |
|
| Run Time | ≤ 3.5 min |
| Injection Volume | 0.2 – 50 µl |
| Gradient | Group 1 |
| Ionisation | ESI positive |
| MS/MS Mode | MRM |
| Additional Info | We recommend to set the scan time to a value that allows to achieve a minimum of 10 data points over the whole peak width. |
| Parameters | Amprenavir, Atazanavir, Darunavir, Efavirenz, Elvitegravir, Indinavir, Lopinavir, Maraviroc, Nelfinavir, Nelfinavir-M8, Raltegravir, Ritonavir, Saquinavir |
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS -
 Internal Standard Mix Anti-HIV DrugsOrder no.: 92844/RUOComponent of the Parameter Set Anti-HIV Drugs, available separately
Internal Standard Mix Anti-HIV DrugsOrder no.: 92844/RUOComponent of the Parameter Set Anti-HIV Drugs, available separately
-
 Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200/RUO
Basic Kit A for 200 Tests - LC-MS/MSOrder no.: 92111/200/RUOPart of the modular system MassTox® Series A
Provides all components required for sample prep and all mobile phases -
 Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000/RUO
Basic Kit A for 1000 Tests - LC-MS/MSOrder no.: 92111/1000/RUOPart of the modular system MassTox® Series A
Provides all components required for sample prep and all mobile phases
-
 6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS
6PLUS1® Multilevel Plasma Calibrator Set Anti-HIV DrugsOrder no.: 92053MassTox® Series A Anti-HIV Drugs in Serum/Plasma – LC-MS/MS