What Benefits Does IVDR Bring to Manufacturers and Laboratories?
Challenges and Opportunities of the New Diagnostics Regulation.
Compared to companies in the pharmaceutical and medical technology sectors, diagnostics manufacturers and medical laboratories had significantly lower regulatory effort. This view has changed since May 2017, when the new IVDR came into effect that is compulsory from May 2022. This new European regulation controls the manufacture of medical products and in-vitro diagnostics. This is many times stricter and involves considerably greater time and effort.
In the past, IVD manufacturers were able to self-declare around 90 per cent of their CE products without an external review of the required documents and records. This review was generally carried out as part of the standard audits by the certification bodies. With the new regulation, the number of certifiers will be significantly reduced, upgraded to the “notified body”, with tighter regulations.
Regulatory experts are therefore predicting a restructuring of the IVD market and laboratory environment. A symposium of the AWMF in February 2020 in Lübeck (Link 1) once again confirmed it: in laboratories there is great uncertainty surrounding the new IVDR. “We have a lack of clear answers and official statements. We do not really know what it means for our laboratory and, above all, how we should best prepare for it. This is a thoroughly unsettling situation,” a participant at the symposium concluded. “We have a lack of statements and guidance from the appropriate bodies to highlight the regulatory tasks for medical laboratories in a transparent and uniform way.”“But, as manufacturers, we also face major challenges to comply with the IVDR 2017,” said Dr. Ralf Fischer, in charge of Regulatory Affairs at Chromsystems and responsible for implementing the new IVDR in the company. The companies are already dealing with additional, not yet conclusively manageable regulatory, personnel and financial costs for implementing the required regulations.


Dr. Ralf Fischer, Head of Regulatory Affairs at Chromsystems
“But, as manufacturers, we also face major challenges to comply with the IVDR 2017,” said Dr. Ralf Fischer, in charge of Regulatory Affairs at Chromsystems and responsible for implementing the new IVDR in the company. The companies are already dealing with additional, not yet conclusively manageable regulatory, personnel and financial costs for implementing the required regulations.
The Regulatory Framework
To date at Chromsystems newly developed methods are comprehensively and carefully validated. The conformity of the background processes are evaluated on a regular basis via external audits, which the notified body carries out on site. “We have realised many audits, including from TÜV Süd, from the US health authorities FDA, the Korean authorities and the Brazilian ANVISA, which all confirmed our conformity in accordance with all individual and country-specific regulations. This won’t change, but there will be additional duties.” Chromsystems had issued the CE declaration of conformity for each product themselves when it had fulfilled the relevant criteria and completed the processes for the development of a CE-IVD. “Soon it will no longer be possible to do it this way,” says Dr. Fischer.
This is because under the new regulation, each kit has to complete a re-classification and re-registration, meaning that self-certification will only be possible in exceptional cases. Instead, virtually each certification is completed via the notified body. This leads to a stricter documentation involving greater time and effort, but also to better monitoring after products are placed into the market. Unique Device Identification (UDI) has been introduced for improved traceability, and is mandatory from 2024: each product will require a UDI number, which will be displayed on the label, amongst other locations, and ensures that the product is easier to trace.
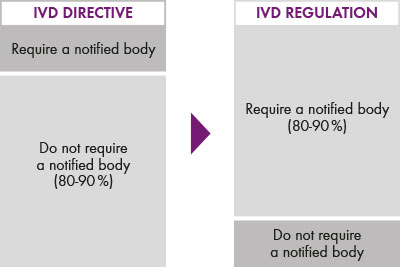

Fig.1: While previously 90 per cent of all IVDs were CE self-declared, and no notified body was involved, the landscape will soon change with the new regulation.
Additional Effort or a Benefit?
Is it worth it, or is it simply more regulatory work? “What may be disadvantageous to us as the manufacturer could well be seen as an advantage for the customer,” says Dr. Fischer. “The additional duties ultimately lead to a rise in quality: more in-depth verification and validation in the analytical and diagnostic area as well as monitoring of the products we have developed, produced and brought to market, for example, will increase.”
A new component is the clinical evidence, an area in which the old regulation had until now set comparatively minimal requirements. Against the backdrop in which treatment decisions for patients are made on the basis of an IVD product, it is understandable that the legislators took action, and that the intended use of a kit now has to be clear and traceable.
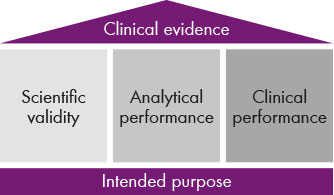

Fig.2: The clinical evidence is made up of three components: scientific validity as well as analytical and clinical performance.
The clinical evidence rests on three pillars: scientific validity, analytical performance and clinical performance (see fig. 2). For scientific validity, manufacturers like Chromsystems have to present a considerable amount of documentation to the notified body which provides evidence on the plausibility of a test: when is it making sense to determine a certain parameter, can I use it to diagnose diseases and, if yes, which ones? Is it making sense to carry out therapeutic drug monitoring for a certain drug and what evidence can I as a manufacturer provide for this? For some parameters, that is easy because there is already a wealth of scientific literature available. The analysis of biogenic amines, for example, for the diagnosis of tumours is documented by a multitude of studies. In other cases it’s a different story, such as for metabolic disorders. “If it’s possible to determine more than 40 amino acids with one assay, then studies on the significance and sensitivity are required for each of these parameters and for each disease that I would like to employ the assay for. This must then also be reflected in the intended use of the assay,” says Dr. Fischer. “This is where the manufacturer specifies what the laboratories can use the IVD test for and what not.”
When there are no studies available in the scientific literature and if a retrospective view of existing data and clinical pictures related to this is insufficient, then the manufacturers must carry out these studies themselves to provide the required evidence. This can be extremely time-consuming in some cases and lead to unacceptably high additional financial costs. “But in the long term it will generally lead to higher quality of IVDs, but likely also to a drop in variety,” Dr. Fischer predicts.
The analytical performance corresponds with what many manufacturers such as Chromsystems have already taken into account in the development of an assay. What is new is that the regulation describes precisely what needs to be tested. “This doesn’t pose a problem since we have been doing this all along anyway. When it comes to clinical performance, it’s a different story.”
The Newborn Screening Example
The newborn screening assay by Chromsystems is categorised into risk class C and requires a range of studies including external performance studies. These studies need to measure around 1500 real patient samples. This must include samples that are positive for a multitude of metabolic disorders such as PKU, MSUD, tyrosinaemia and all those which are to be specified in the intended use.
Then the cut-off value for each amino acid and each disease must be defined in such a way that the test guaranteed to deliver 0 per cent false negative results while at the same time delivering a minimum of false positives. (see fig. 3). A second tier test then provides the confirmation analysis in the case of a positive test.
If a laboratory wants to use the kit or the analysis method for further applications other than those that are listed in the kit manufacturer’s intended use, then the laboratory is responsible for the medical and diagnostic performance and for the documentary obligations. The test then becomes a lab developed test (LDT).
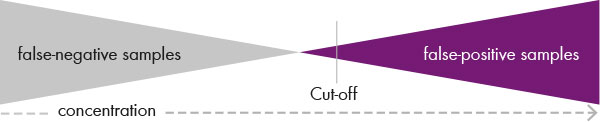

Fig.3: In newborn screening, the cut-off value must be defined such that the test delivers
0 % false negative samples while also delivering as few false positive samples as possible.
Pros and Cons of the New Regulation
The effort for the manufacturer leads to an IVDR compliant kit requiring a development time of between 1 and 3 years. Faster processes are unrealistic and are not conceivable in the future. The additional efforts required by the IVDR 2017 also entails a considerable increase in financial expenditure before the products are market ready. On the upside, this time and effort does provide a maximum of security and reliability. Post-market surveillance in particular is probably a quality feature that laboratories are unable to realise. A manufacturer that is globally represented on the market can recognise much faster if, for example, a newly approved drug or metabolite will lead to re-evaluation and monitoring of the analysis method. “We need to react quickly, adjust the assay accordingly and inform the customer of the assay as quickly as possible. In-house methods are disadvantageous at times. They may notice interferences only later, while customers at Chromsystems have already switched to an improved product. This enables problems to be solved before they occur in the lab,” explains Dr. Fischer.
Significance for Medical Laboratories
Meanwhile, medical laboratories also have to deal with a lot of work, especially for the LDTs. Validation and verification work is on the rise. Fundamentally, they have the same regulations to fulfill as a manufacturer of CE-IVD products, as set down in appendix 1 of the regulation (Link 2). For self-developed tests, this means additional validation tasks as well as providing proof of the method in terms of diagnostic and analytical evidence. The time and effort spent on documentation will definitely increase. This is alone due to the fact that labs need to document why a commercially available IVDR kit is not being used even though it has the same performance characteristics as the in-house method. “Discussions on this are definitely happening in all laboratories. Many laboratories with in-house methods will switch to manufacturer kits due to the soon-to-be-mandatory tasks of the new IVDR because it will be cheaper for them in the long run. Others are waiting and seeing,” says Dr. Fischer.
Last Update 9th of June 2021
Further Information

