LC-MS/MS in Drugs of Abuse Testing for Target Screening and Quantitative Confirmation
Drugs of abuse testing is performed to identify drug abuse, to monitor someone with a substance abuse problem, or to detect drug intoxication and overdose. The identification of drug abuse in biological samples can be used in court as scientific evidence, and it can help to improve the quality of clinical management during emergencies. One of the most common screening methods used for the detection of drugs in urine and other matrices are immunoassays. They are convenient, but they have their limitations: results are often class-specific and cannot be attributed to a specific drug or drug metabolite. Antibodies are also susceptible to cross-reactivity with structurally related and unrelated compounds, which increases the risk of false-positive results. This is why data gathered by immunoassays are considered as presumptive.
GC-MS vs LC-MS/MS
An immunoassay requires a second analytical procedure to confirm the quantitative determination, and this is usually performed by either GC-MS or LC-MS. The mass spectra obtained from the GC-MS can be compared with large databases, enabling the unknown abusive drugs to be identified – this is one of the reasons why GC-MS has been the gold standard in drugs of abuse testing for many years. However, most compounds of interest need to undergo a chemical derivatisation to make them more volatile and compatible with GC analysis – without derivatisation, GC-MS generally offers poor peak shapes, lower resolutions and reduced sensitivities. However, undertaking more sample preparation steps also increases the risk of errors, and acidic derivatisation can be prone to uncertainties, such as the reagent quality, the presence of interferences, and variable lab conditions.
In contrast, LC-MS is ideal for polar and non-volatile molecules such as those analysed in drugs of abuse testing. An efficient separation and ion generation can be achieved without derivatisation and LC-MS generally requires less sample preparation than GC-MS. Among the different mass spectrometry platforms, triple quadrupole mass spectrometry with multiple reaction monitoring (MRM) is the most commonly adapted technique.
LC-MS/MS – From Theory to Practice
A laboratory tested a commercial LC-MS/MS assay (MassTox® Drugs of Abuse Testing, Chromsystems) and compared it with GC-MS, with a focus on routine analysis [1]. The sample prep for the amount they routinely deal with usually takes 6 hours (excluding hydrolysis), but by using the commercial assay, the lab was able to reduce the time down to 2 hours. The switch from GC-MS to LC-MS reduced the resources required for the sample prep, and the sample volume required for the sample preparation was also significantly lower (see Table 1).
| GC-MS in-house methods | LC-MS/MS Assay | |
| Sample Prep Time (excluding hydrolysis) | 6 h per day | 2 h per day |
| Sample Volume | 1000-5000 µl | 50 µl |
| Run Time | 10-15 min | 15 min |
Table 1: Comparison of GC-MS with LC-MS/MS
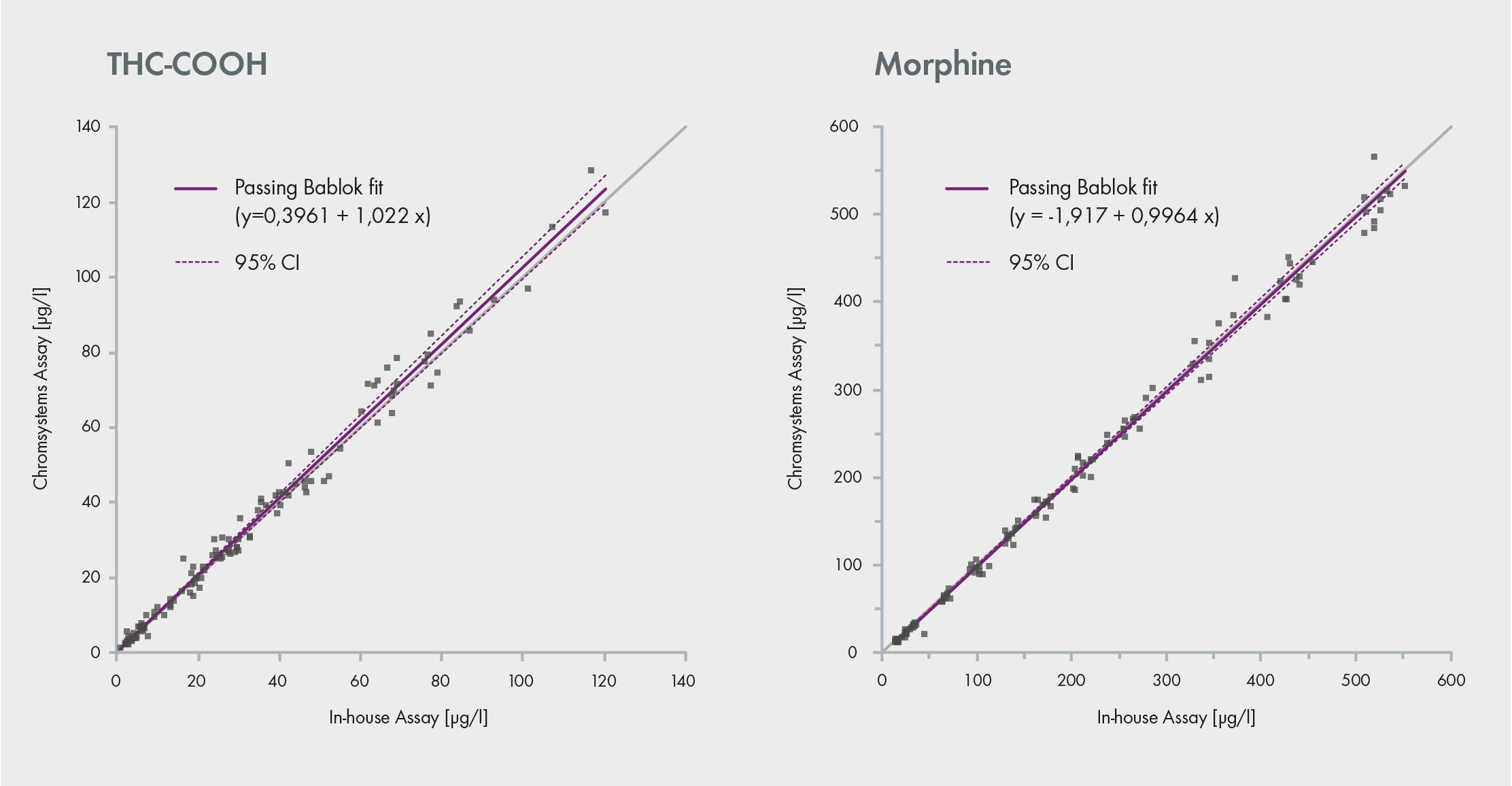

Fig. 1: Comparison of a commercial assay with an in-house method. Both assays provide similar data
The lab also conducted comparative analysis between the commercial assay and an in-house LC-MS assay used by an external accredited laboratory. The values obtained correlate very well with each other across a range of concentrations demonstrating a high accuracy, as showcased by the linear results. Therefore, the commercial assay (Chromsystems) is suitable for replacing LC-MS/MS in-house methods and allows for the target screening and/or quantitative confirmation of 106 drugs in a single run (Fig. 1). Proficiency testing schemes from GTFCh and RfB, in which the assay has been used, also confirmed its accuracy [1].
100% Hydrolysis – 0% Doubt
In the human body, many drugs undergo glucuronidation, which requires an enzymatic or acidic hydrolysis prior to the analysis – a challenge for many assays. Enzymatic hydrolysis varies in its effectiveness depending on the drug and the enzyme [2]. Erratic quality assurance results for codeine – one of the more difficult to hydrolyse compounds – are considered to be based on an incomplete hydrolysis [3]. Consequently, some papers recommend the use of an acidic hydrolysis, however, this can degrade both opioids and other substances [4]. Furthermore, this approach can convert oxycodone to oxymorphone, and codeine to morphine by demethylation, which increases the risk of false-negative or false-positive results. To overcome this drawback, our lab has developed an enzymatic hydrolysis process that is effective for hydrolysing all glucuronides within 2 hours. This has been achieved by using a carefully selected enzyme that ensures a complete and selective hydrolysis of all 106 drugs that are covered in the assay, including codeine. The effectiveness has been demonstrated by measuring the hydrolysis of several substances over time: Easy-to-hydrolyse glucuronides become fully hydrolysed quickly, while others, such as codeine, require longer. After 2 hours, the hydrolysis is complete for all the compounds (Fig. 2).
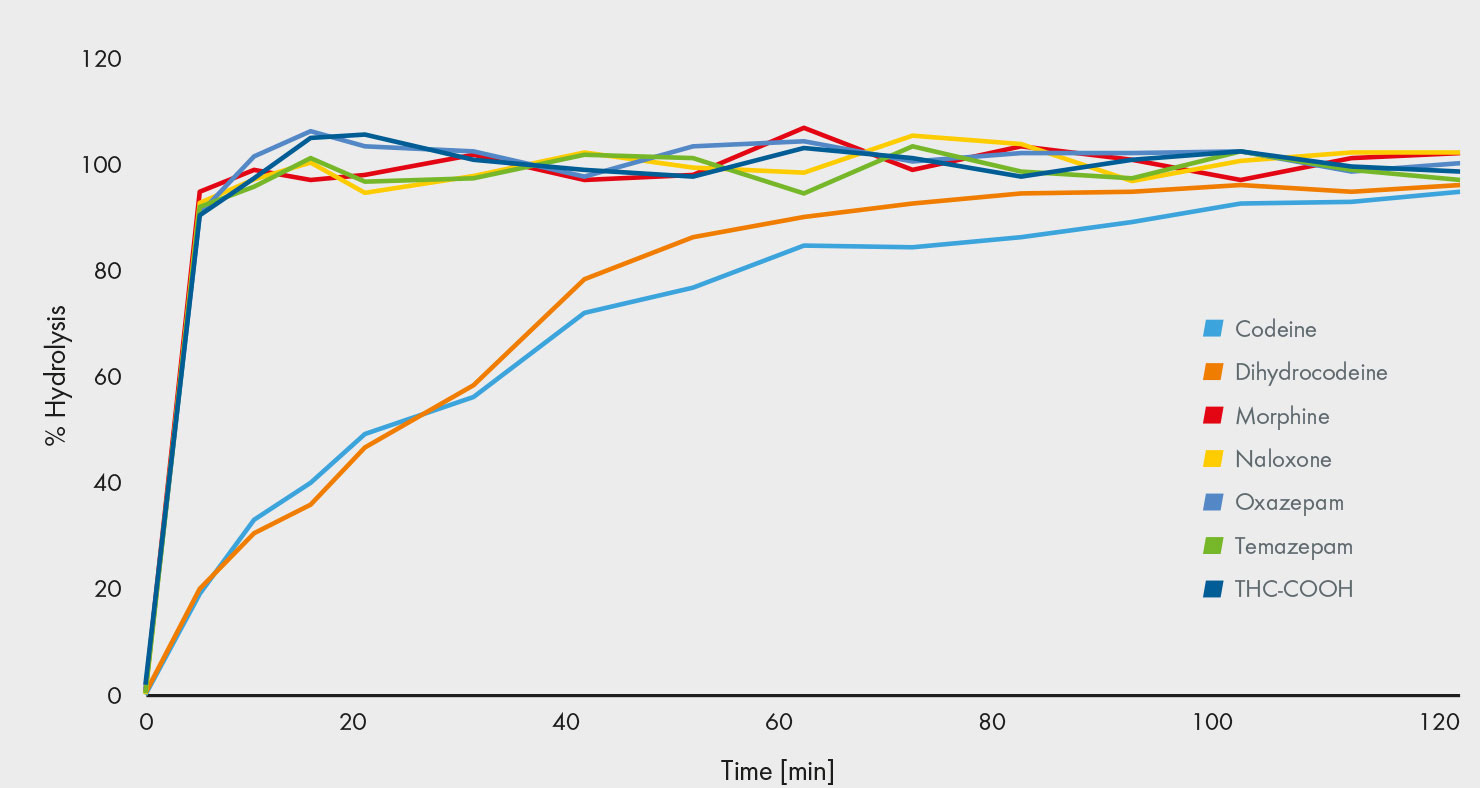

Fig. 2: After 2 hrs, hydrolysis is complete, including difficult to hydrolyse drugs such as codeine.
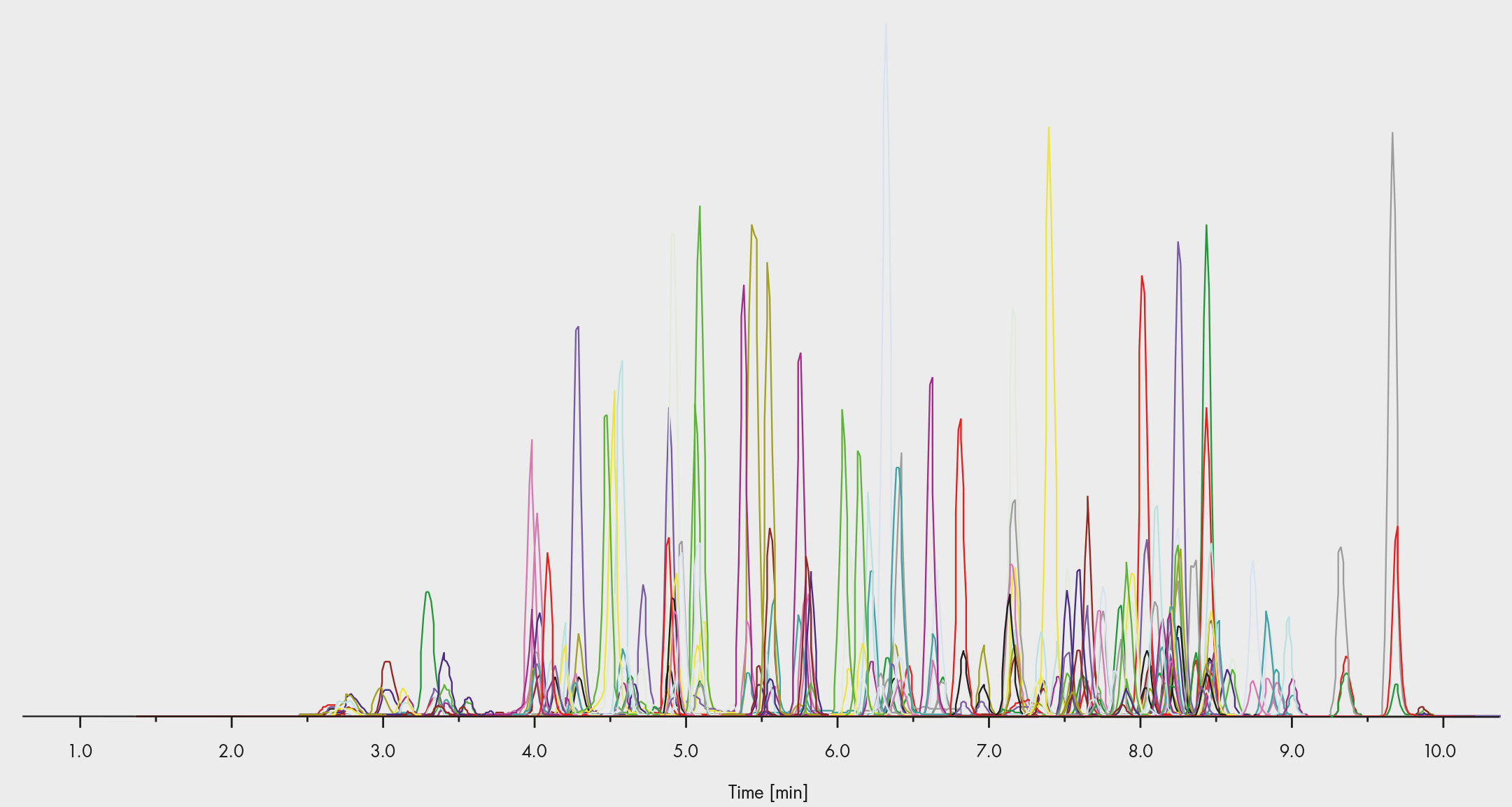

Fig. 3: Chromatogram of the commercial assay – more than 100 drugs can be tested within a single run
Target Screening and Confirmation in One Run
Immunoassays often require an alternative method to confirm the results, and this is how many organisations have laid out their drug abuse testing schemes. However, LC-MS/MS has an accuracy and selectivity that is a sufficient to do both in one step. This is why commercial assays, such as those from Chromsystems, enable the target screening and quantitative confirmation of more than 100 drugs in a single run (Fig. 3), including benzodiazepines, opioids, booster, and Z-drugs. In the case of a positive result, the quantification can be evaluated straight away from the same peak. Labs might find this option in drug of abuse testing appealing, as it reduces the resources required without compromising on the accuracy.
References:
[1] Geffert et al., Validation of a New LC-MS/MS Assay for the Analysis of Drugs in Urine and Comparison with Established Analytical Methods (GC-MS and LC-MS/MS): Advantages for daily Laboratory Routine. GTFCh Symposium 2019.
[2] Wang P et al., Incomplete Recovery of Prescription Opioids in Urine using Enzymatic Hydrolysis of Glucuronide Metabolites. J. Analytical Toxicology, (2019), 571-575.
[3] Hackett LP et al., Optimizing the hydrolysis of codeine and morphine glucuronides in urine. Ther Drug Monit., (2002), 652-657.
[4] Opiate & Benzodiazepine Confirmations: To Hydrolyze or Not to Hydrolyze is the Question. J. of Appl. Lab Med., (2018), 1-9.
Article originally published in:
Clinical Laboratory International, September 2019, p. 24-25
