IVDD and IVDR - your questions, our answers
We collected on this page the most frequently asked questions (FAQs) around Chromsystems and the IVDR.
Do you have any further questions? Would you like to get to the bottom of any uncertainties? Please feel free to contact either your sales representative or technical support at the e-mail support@chromsystems.com. You can also reach us via a general inquiry on the website.


What are IVDD and IVDR and what is the difference between them?
In vitro diagnostic medical devices (IVD) used to be subject to EU Directive 98/79/EC (IVDD). The requirements defined in the IVDD were implemented by the EU member states in their national legislation. This previous directive was replaced by the new EU Regulation on in vitro diagnostic medical devices (IVDR) and entered into force on 26 May 2017. Since this is not a directive, but instead an EU-wide regulation, it applies directly in all member states of the EU.
The main changes of the IVDR are summarised below:
Documentation
Stricter requirements for documentation, especially for the technical documentation and post-market surveillance.
Classification
New classification: Class A (low risk) -> Class D (high risk)
Clinical evidence
Stricter requirements for clinical evidence
Sampling process
New sampling procedure for IVD products on the market and review of technical documentation
New requirement
Designation of a person responsible for regulatory compliance (PRRC) in the company
Notified body
Increased surveillance of the company by the notified body
UDI
Unique identification number (Unique Device Identification - UDI) for every medical product
EUDAMED
Europe-wide database for enhanced transparency and traceability
What do the CE and IVD markings mean? For example on the bottles?
The CE symbol, for example on a label or instruction manual, is not a quality seal, but instead a marking which may only be applied by the manufacturer or its authorised representative. It indicates that it meets the requirements of the EU for in vitro diagnostic medical devices.
In the case of CE conformity through the notified body, the respective four-digit number is also stated.
The IVD marking on the product is not a certificate, but merely indicates that it is an "in vitro diagnostic medical device", a medical product, which as reagent, calibrator, control material, kit, is intended for in vitro examination of samples from the human body for diagnostic purposes, including blood and tissue samples.
What are the deadlines for manufacturers under IVDR?
A distinction must be drawn between two cases:
New or modified products:
All products developed and brought into market from May 2022 onwards as well as assays with significant changes must be compliant with the IVDR. This is independent of the product class.
Existing products:
Since the beginning of 2022, new transitional periods (Regulation 2022/112/EU) have been announced for existing products, which depend on the classification of the product.
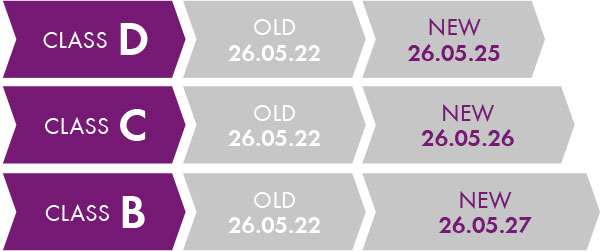

Transitional periods of Class B to Class D products.
Important!
Products in the laboratory may continue to be used legally after May 2022, since they had already been distributed by Chromsystems for the first time.
There is no transitional period for Class A; only in the case of A sterile, which is May 2028. An exception are products that already had a certificate under the IVDD , such as newborn screening assays for the determination of phenylketonuria. A transitional period applies here until 26 May 2025. Chromsystems generally offers IVD products in the B and C classes. Examples:
Class B
Crosslinks, porphyrins, homocysteine, TDM (excluding immunosuppressants), DoA and occupational medicine, vitamins, CDT, ETG, Glu, MDA and Q10
Class C
Biogenic amines, newborn screening, immunosuppressants, steroids, cortisol, amino acid analysis (AAA) in plasma and urine
Are all Chromsystems products IVD-compliant from May 2022?
Despite the extended transitional periods, we retain our focus on implementing the IVDR as quick as possible. A complete list of our products currently under the IVDR you can find here.
Since self-certification for Class B and C products is no longer possible, manufacturers are dependent on the notified bodies. Consequently, a successive conversion of all products takes place.
Important!
Conformity and availability of all Chromsystems products is guaranteed at any time due to the transitional periods.
Who is the notified body for Chromsystems?
The notified body is TÜV-Süd Product Service GmbH, as is stated on the product labels with the corresponding identity number CE0123.
What is the current status at Chromsystems?
As an IVD manufacturer, we have made an enormous effort to achieve the implementation of the new IVD Regulation (IVDR [EU] 2017/746). This includes comprehensive investments in resources in the regulatory environment. Despite the extended transitional periods, we retain our focus on implementing the IVDR as quickly as possible.
Currently, several, but not all, products are IVDR-certified. The background is that despite close cooperation and coordination with the notified body, the requirements of the IVDR are complex and extensive.
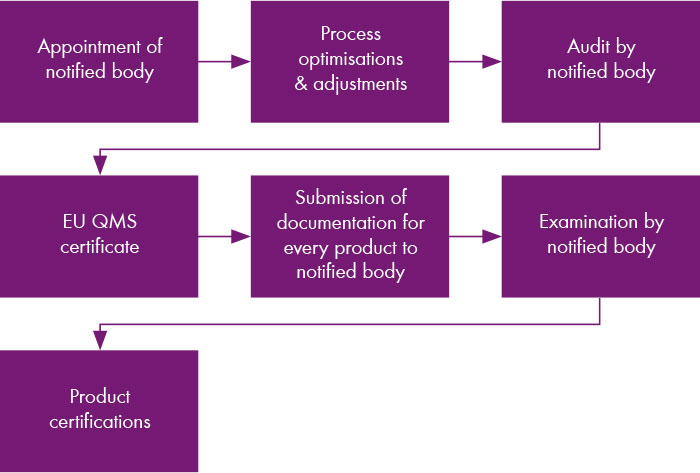
In the first on-site audit, the overall process of the company and the first products were audited. The company as well as five assays were certified in July 2022. Currently, the documentation for all other products is being successively prepared and transmitted to the notified body. However, Chromsystems is also using the conversion to IVDR for product optimisation.

Can delivery delays be expected due to the conversion to IVDR?
No. Delivery problems are not expected for Chromsystems products within the specified framework and timelines of the new IVD regulation.
Since products produced before the certification can be used for three years or longer, depending on the classification, you may receive IVDR and IVDD products in parallel in the transitional period up to May 2028. It is possible that the production of individual components occurs partially before and partially after upload of the the documentation to the notified body and therefore CE- and CE0123-marked products are included in one shipment. However, since the composition and properties of the products are not changing, they can be used and combined with each other without any restrictions.
Can IVDD kits be used after May 2022?
Yes. Existing products in the laboratory may continue to be used legally after May 2022, as they had already been put onto the market.
Even if several products do not possess the new CE-IVDR certificate in the transitional period, they can still be used in the diagnostic routine as IVD/CE within the permissible scope, since they are already in the commercial chain and been placed on the market for the first time. These products will be available until all product groups have been certified by the notified body. Our products can be used until the end of their product life.
Will all Chromsystems products be transferred to the new IVDR?
Based on current information, all kits at Chromsystems will be transferred to the new IVDR and within the permitted IVDR transitional periods.
We ensure that all products will be available without interruption, however, no statement can currently be made as to when particular kits will be certified. Despite close cooperation and coordination with the notified body, the IVDR requirements are so complex and extensive that stating periods for the conversion of individual kits is currently not possible.
When do I have to implement the IVDR in the laboratory?
In general, laboratories must adhere to ISO 15189. Users only have to implement the IVDR if they use lab-developed tests (LDTs). The Regulation 2022/112 extended the transitional period for this procedure to May 2024.
To what extent are the kits in newborn screening a special case? Are they already IVDR-compliant due to the CE0123 marking?
The assays for newborn screening (order no.: 55000/57000) were classified under the IVDD such that the conformity assessment had to be performed by a notified body (classified as Class B product according to Annex II and thus conformity according to Annex IV of the IVDD). Consequently, these product series were also previously exempt from self-certification and were certified by TÜV Süd. Consequently, they had already been treated very similar in the past to what is required by the IVDR. Due to the TÜV certification, CE0123 had always been printed on these product labels.
These products are certified until 25.5.2025 by TÜV and may also be used and sold as such.
How can I tell if the product is IVDR-authorised?
With the exception of the newborn kits, an IVDR-authorised product can be recognised based on the CE0123 marking, which can be found on the labels and in the instruction for use (IFU). In addition, the CE declarations are available for download on the website and show whether a product is distributed under IVDD or IVDR.
Which Chromsystems products are already IVDR-certified?
What can I do if I have further questions?
In you have additional questions, please contact your sales representative or our technical support staff at support@chromsystems.com or send a general inquiry directly from the website.
